










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Purpose: Monkeypox, an orthopoxvirus with zoonotic origins, has emerged as a global health concern due to recent outbreaks outside of endemic regions. Pregnant and lactating individuals are particularly vulnerable to potential complications, emphasizing the need for targeted prevention and management approaches.
Methods: This review explores the clinical presentation, impact, diagnostic methods, and treatment options for monkeypox in pregnant and lactating populations, with a focus on safety, effectiveness, and prevention strategies.
Results: The review synthesizes current literature on monkeypox, specifically addressing supportive care, antiviral therapy, natural remedies, vaccination, and infection control practices. Special considerations for maternal and fetal health are discussed, along with preventive guidelines to minimize transmission risks. Supportive care is the primary management approach, with antivirals like Tecovirimat and Brincidofovir considered in severe cases.
Conclusion: While vaccination offers preventive protection, strict hygiene and protective measures are crucial in healthcare and community settings. For lactating mothers, expressed milk and alternative feeding methods can mitigate transmission risks. Effective management of monkeypox in pregnant and lactating individuals requires a balanced approach that prioritizes maternal and infant safety. Further targeted research is needed to identify the specific impact of the virus on pregnancy outcomes and breastfeeding practices.
Monkeypox (MPX) has become an emerging global health concern as a significant zoonotic infection, especially following recent outbreaks in regions outside its endemic areas. The monkeypox virus (MPXV) is one of the zoonotic viral illnesses which can cause severe health issues and affect multiple populations in different ways (1). It is a viral zoonotic disease caused by the MPXV, which is part of the Orthopoxvirus genus in the Poxviridae family (1). The virus is primarily transmitted to humans through direct contact with animals (particularly rodents or primates) or through human-to-human transmission via respiratory droplets or contact with bodily fluids (1). The recent global outbreaks of MPX, particularly in 2022, have raised significant public health concerns, especially regarding the risks to vulnerable populations, including pregnant individuals (1).
While MPX has been known for decades, it was primarily confined to central and west Africa, where it occurs sporadically. However, the 2022 global MPX outbreak, which spread to countries across multiple continents, highlighted the need for greater awareness and understanding of its transmission, clinical manifestations, and potential risks, particularly in pregnancy (2).
The recent resurgence, coupled with transmission patterns extending beyond traditional geographic boundaries, has underscored the urgent need for comprehensive reviews on monkeypox prevention and management, particularly in vulnerable populations (3,4).
The resurgence of monkeypox, caused by the monkeypox virus (MPXV), has raised global concerns, especially regarding its impact on vulnerable population s like pregnant and lactating women. With symptoms resembling smallpox but often less severe, monkeypox spreads primarily through close contact, but recent cases have demonstrated potential human-to-human transmission, emphasizing the need for focused prevention and management strategies (5). Pregnant women represent a particularly high-risk group due to possible transmission to the fetus, complications like premature birth, and even miscarriage. This risk has spurred research on effective preventative and therapeutic interventions tailored to this demographic (6). Therefore, exploring safe, accessible, and comprehensive preventive and management measures for monkeypox during pregnancy and lactation has become a priority in global health research (7). In the current literature, there are limited studies on the effect of MPX in pregnancy and lactation (2).
Varying the physiological changes in terms of immunity and hormonal changes, the pregnant women and breastfeeding mothers are among the most vulnerable groups when it comes to infectious diseases. According to previous literatures and evidences, the viral infections such as Zika, SARS-CoV-2, and influenza during pregnancy can manifest serious health threats for both mother and fetus especially during outbreaks (8,9). These infections also lead to increase maternal morbidity and severe unfavourable pregnancy outcomes, such as miscarriage, premature birth, and congenital defects. These potential consequences bring a heightened urgency to understanding how monkeypox impacts pregnancy and lactation, as well as identifying specific preventive measures and management strategies for this population (10).
Studies suggested that MPX, like other viral infections, induces a pro-inflammatory cytokine response, which may contribute to adverse pregnancy outcomes. For instance, elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), seen in SARS-CoV-2 and Zika virus infections, are also implicated in MPX pathogenesis (11,12). Additionally, immune modulation mechanisms such as type I interferon suppression and placental immune dysregulation in Zika and MPX infections warrant further investigation.
Moreover, there are some teratogenic viral infections such as cytomegalovirus (CMV), rubella, and Zika virus, all of which are known to cause congenital syndromes. These viruses disrupt fetal development through mechanisms such as direct neural invasion (Zika), placental dysfunction (CMV), and inflammatory responses leading to congenital defects (rubella) (13). While MPX is not currently classified as a teratogenic virus, its potential to cause fetal complications warrants further study. This expanded analysis identifies key patterns in viral pathogenesis that may inform future research.
Therefore, while evidence remains limited, the potential for adverse outcomes, including preterm birth and maternal complications, calls for a focused examination of monkeypox‘s effects on these populations. This review aims to consolidate current knowledge on monkeypox with a focus on pregnancy and lactation. We explore the virus’s clinical manifestations, risks associated with maternal and fetal exposure, and the nuances of disease progression in pregnant individuals. Additionally, we examine diagnostic measures, treatment options tailored to minimize harm during pregnancy, and preventive strategies. Given the distinct challenges associated with managing infectious diseases in these groups, understanding how monkeypox presents and progresses in pregnancy and lactation is crucial for providing safe and effective care. Therefore, though the aim of the review is to provide healthcare professionals with practical insights and guidance on mitigating risks associated with monkeypox during pregnancy and lactation. By providing a concise review on the prevention and management of MPX in pregnancy and lactation, study aimed to contribute on foundation for future research and policy development on managing monkeypox in these high-risk groups (14,15).
Similar to smallpox, monkeypox is one of the forms of zoonotic diseases caused by the monkeypox virus which is a member of the Orthopoxvirus genus. (1) MPX, previously known as Monkeypox, was first reported in humans in 1970 in the Democratic Republic of Congo. It is a zoonotic disease caused by monkeypox virus (MPXV), an orthopoxvirus of the poxviridae family (1). It is a double stranded DNA virus with a large genome encoding 191 proteins. It was identified during 1958 during outbreaks in research monkeys, the virus primarily circulates in Central and West Africa and has been associated with animal reservoirs, including rodents and primates (16). Human cases were first documented in the Democratic Republic of Congo in 1970, and the disease has since become endemic in various African regions (17).
It is a double stranded DNA virus with a large genome encoding 191 proteins. There are two main clades (subgroups), which are Clade I and Clade II. Clade I, which reportedly causes more severe illnesses is endemic in central African countries whereas Clade II, which causes a milder self-limiting disease is endemic in West African countries. Clade II is further classified into IIa and IIb by Happi et al. (2022) (1). In 2022, MPXV Clade IIb spread worldwide affecting 99,000 people across at least 118 countries (18).
Prior to 2022, MPX cases outside of the African continent were rare. They were related to international travels or importation of infected rodents. In 2022, there was a global outbreak of clade IIb largely spread through sexual transmissions and contained in the sexual networks of individuals who are gay, bisexual, and other men who have sex with men (1,18).
MPX can be transmitted from animal to human via direct contact with infected fluids, lesions, bites, scratches, contaminated fomites such as linens and clothing, respiratory aerosol and droplets (1,18). The MPX reservoir is recognized to be arboreal rodents in African forest, however the full host range of monkeypox is not fully known. It is also documented that the Asian orangutans are among the hosts of MPXV (19). Human to human spread occurs with direct contact with infected skin lesions, mucosa, bodily fluids and blood. There is evidence that states that human to human transmission can occur during the asymptomatic incubation period prior to showing any symptoms of harbouring the virus. This had contributed to the spread of the virus (18).
Transmission occurs via contact with infected animals, human-to-human transmission, or contaminated materials. Human-to-human transmission primarily involves respiratory droplets, direct contact with lesions, or bodily fluids, which has implications for close-contact settings, including households (20). Monkeypox usually takes seven to fourteen days to incubate before symptoms appear.
Moreover, people who infected with monkeypox may suffer the symptoms such as during initial phase, people may suffer fever with chills and rigor, malaise, headache, myalgia which is followed by the emergence of a distinctive rash. Frequently compared to smallpox, the rash develops from macules to papules, vesicles, and pustules before crusting and recovering over a few weeks (9). Lymphadenopathy, a distinctive feature of monkeypox, often sets it apart from smallpox and can aid in differential diagnosis (9).
Though there are some similarities in terms of clinical manifestation among monkeypox and smallpox, monkeypox is generally less severe, with a lower-case fatality rate. The virus infects resident immune cells and local lymph nodes and rapidly replicates. This enables the virus to spread through the lymphatic system and the bloodstream and disseminate to multiple organs. Generally, MPX infection is self-limiting and mild. However, it can be present as a severe illness especially in immunocompromised patients which includes pregnancy (18,19).
However, serious side effects have been documented, including respiratory distress, encephalitis, and secondary infections, especially in immunocompromised people and those with concomitant illnesses (21,22).
Monkeypox infection generally presents with the initial set of nonspecific symptoms, followed by a characteristic prodromal symptom such as fever, malaise, lymphadenopathy, and rashes through the stages of macules, papules, vesicles, and pustules ultimately crusts before healing. These skin lesions are usually concentrated on the face and extremities, though they can appear throughout the body (3,4). The characteristic clinical features of MPX are the painful skin lesions that developed over 2 to 4 weeks. These skin lesions progress uniformly through 4 well defined stages namely macules, papules, vesicles and pustules which crusts and desquamates in the final stages. Patients can be infectious from the incubation period until all the skin lesions have epithelialized (1,18).
The prodromal symptoms and skin lesions are generally similar to that of non-pregnant patients. Pregnant individuals may experience more severe MPX infections due to immunological adaptations that occur to prevent fetal rejection. Pregnancy-induced immune modulation, which has been observed in infections like influenza and COVID-19, increases susceptibility to severe viral illnesses, leading to a heightened risk of complications. While the clinical presentation of MPX in pregnancy largely mirrors that of non-pregnant individuals, pregnant patients may experience increased fatigue and a prolonged recovery period (2,18). This altered immunity can increase susceptibility to certain infections or exacerbate the severity of viral illnesses, as seen in diseases like influenza and COVID-19 (8,22). Though data on MPX in pregnancy remain limited, case reports suggest an increased risk of adverse outcomes, including miscarriage, preterm labor, fetal loss, and stillbirth. There have been documented instances of MPX virus detection in placental tissue, amniotic fluid, and breast milk, raising concerns about vertical transmission. A case study from the 2022 European outbreak reported a fetal demise associated with intrauterine MPX infection, but such occurrences appear to be rare. Despite this, MPX infection warrants careful prenatal monitoring to mitigate risks and manage potential complications (10).
According to Bunge et al. (2022), based on the data from broader surveillance studies and systematic reviews when discussing fetal outcomes and maternal MPX infection, fetal outcomes in maternal monkeypox (MPX) infection may include adverse events such as miscarriage, preterm labor, stillbirth, and, in rare cases, congenital monkeypox. Evidence suggested that MPX virus can be detected in placental tissue, amniotic fluid, and breast milk, raising concerns about vertical transmission (4).
A case study from the 2022 outbreak in Europe described a pregnant woman who developed MPX, and her fetus had shown signs of intrauterine infection leading to intra uterine death of the fetus. This was an isolated case reported. Lactating mothers who have been diagnosed with monkeypox infection was advice to take caution and advised to not breastfeed her baby until she has fully recovered from the infection to prevent transmission to the baby. Evidence from case reports and similar viral infections indicates that monkeypox has the potential for transplacental transmission, which could impact fetal development. Adverse fetal outcomes have been documented in certain cases, including miscarriage, preterm labour, and stillbirth, although more research is needed to fully understand these risks (14,15). The impact of MPX during pregnancy and lactation had shown potential for pulmonary hemorrhage, epistaxis, and impaired coagulation parameters in animal studies. Long term effects on humans were yet to be documented. However, effects of MPX during pregnancy may lead to preterm births and fetal growth restrictions which may indirectly have long term effects on the development of the baby (23). Given these risks, pregnant individuals with MPX may require more intensive medical supervision. Severe cases may necessitate hospitalization to manage complications such as dehydration, respiratory distress, or secondary infections. Supportive management, including fluid resuscitation, fever control, and symptom relief, is essential to improving maternal and fetal outcomes. However, limited data make it difficult to determine precise risks, but early identification and supportive management are crucial in preventing adverse outcomes (23).
According to previous literatures, while reports of fetal demise and congenital MPX were concerning, they were based on isolated case reports and did not establish a direct causal relationship. Instead, studies highlighted a possible association that required further epidemiological research. This clarification ensured that the readers interpreted these findings appropriately and avoid overstating the evidence (9).
MPX is primarily transmitted via direct contact with infectious lesions, bodily fluids, or contaminated materials. Respiratory droplet transmission is also possible, particularly with prolonged exposure. In pregnancy, vertical transmission remains a concern, although the precise risk level is unclear due to limited data. However, given the potential for congenital infection, pregnant individuals should take extra precautions to avoid exposure. The risk of transplacental transmission, while not fully understood, has been observed in similar viruses within the Orthopoxvirus genus, suggesting that monkeypox might similarly impact the developing fetus if the mother is infected during pregnancy (16,21).
The placenta can play a crucial role in preventing or facilitating vertical transmission of certain viruses. However, in some viral infections, the placenta may not provide sufficient protection, potentially allowing the virus to reach the fetus. In cases where vertical transmission occurs, the fetus may be at risk of severe illness or developmental issues, although further research is needed to understand these risks in monkeypox infections specifically. Given the potential for severe outcomes, healthcare providers must remain vigilant in monitoring and supporting pregnant patients with monkeypox to manage complications as they arise (17).
Given that MPX is an orthopoxvirus, it may share transmission mechanisms such as transplacental infection, placental inflammatory responses, and direct fetal viral invasion. Variola virus, responsible for smallpox, has been shown to cross the placenta, leading to fetal demise and congenital infections (24). Similarly, vaccinia virus, used in smallpox vaccines, has been associated with fetal vaccinia in rare cases when administered to pregnant individuals (25). These insights suggested that MPX may have the potential for vertical transmission, although robust epidemiological data are lacking.
Lactating mothers with monkeypox (MPX) encounter unique challenges due to the potential risk of transmitting the virus to their infants. Although MPX spreads mainly through direct contact with lesions or bodily fluids, there have been reports of the virus being present in breast milk, though evidence is limited. As a result, breastfeeding may pose a transmission risk, especially if the mother has active lesions on her breast. However, current data on the presence of the monkeypox virus in breast milk remain scarce. For lactating mothers with monkeypox, alternative feeding methods may be safer if the mother has active lesions. Mothers should practice strict hygiene and ensure proper sanitization to reduce exposure risk (14). Guidelines generally advise lactating mothers with active MPX lesions to temporarily suspend direct breastfeeding and consider alternative feeding methods, such as expressing breast milk. This approach allows the infant to continue receiving the immunological and nutritional benefits of human milk while minimizing the risk of direct viral exposure. However, strict hygiene measures, including sanitization of breast pumps and feeding bottles, must be followed to prevent potential contamination (3,4,8,9).
Beyond the risk of viral transmission, MPX can impact lactation through systemic illness effects. Fever, dehydration, and inflammation may contribute to reduced milk production. Additionally, painful skin lesions, especially if located on the chest, can make breastfeeding physically challenging. In such cases, lactating mothers may require additional support, including hydration, nutritional supplementation, and pain management, to maintain lactation (10,15). Given the lack of comprehensive data on monkeypox in lactating mothers, further research is essential to establish clear guidelines for safe breastfeeding practices and to assess potential risks for the infant. Until more data are available, healthcare providers must weigh the benefits of breastfeeding against the possible transmission risks in each case.
At present there is no specific pathway by which MPXV traverses the maternal-placental-fetal barrier and infects the fetus. There are many pathways that might be involved in the ability of MPXV to invade placental trophoblast cells (26). MPXV do not have cell-specific receptors facilitating cell tropism, unlike most other viruses that has distinct cellspecific strategies for cell entry and replication within host cells (27). MPXV may reach the fetus via the hematogenous route, entering the intervillous space from the uterine spiral arteries, binding to trophoblast cells, and consecutively infecting syncytiotrophoblasts, cytotrophoblasts, and fetal blood cells. Direct transfer via genital lesions is a strong possibility. Placental pathology on electron microscopy revealed necrotic villi, fibrin deposition, and virions at various stages of assembly (28).
Cytopathic effects like shrinking of the cells or shape changing of the cells in human syncytiotrophoblasts have been observed in placenta infected with vaccinia virus (29). It is believed that MPXV might breach the maternalplacental- fetal barrier via viral fusion with trophoblasts, whereby the viral capsid proteins adhere to target cellsurface receptors. This leads to structural changes in the viral capsid, causing internalization of viral DNA through fusion with syncytiotrophoblast membrane. Once inside, the viruses can replicate and cause host cell damage directly (cytopathic effects) or secondarily to local inflammatory and immune reactions from the host (30). Once the placental barrier is breached, MPXV might be able to infect multiple placental cells reaching the fetal bloodstream eventually.
While MPX DNA had been detected in various bodily fluids, including saliva and skin lesions, its presence in breast milk remains unconfirmed (15). Expressed breast milk from asymptomatic patient is better avoided or the breastfeeding is delayed. The transmission may occur from lesions on or around the breast tissues. Most of the transmission may occur due to direct contact with lesions on the mother rather than the breast milk. Study done in 2021 mentioned that the newborns separated from their mothers who were infected with SARS-CoV-2 had higher mortality from being denied breastmilk than from contracting the virus from mothers who were infected (31,32). To avoid inadvertently exposing an infant to MPXV, the baby can be fed pasteurized donor human milk or infant formula. Given the similarities to other orthopoxviruses, there was a theoretical risk of transmission, particularly if active lesions are present near the breast. However, no epidemiological studies have conclusively demonstrated breastfeeding-related transmission. Therefore, due to the lack of direct evidences, the need for further investigations are crucial.
Effective diagnosis and assessment of monkeypox are crucial for managing the disease, especially in highrisk groups such as pregnant and lactating individuals. PCR testing remains the primary method for detecting monkeypox virus, while serology and antigen detection offer additional information. Monitoring organ function and fetal well-being is essential, particularly in severe cases. Proper infection control measures must be observed in healthcare settings to prevent transmission.
Investigating the characteristics of monkeypox in pregnant and lactating women includes understanding its epidemiology, transmission pathways, and clinical outcomes. The virus can spread via respiratory droplets, bodily fluids, and skin lesions, while vertical transmission to the fetus is possible, although not yet fully understood (6). In cases of maternal monkeypox infection, studies indicate an increased risk of severe disease progression, leading to adverse pregnancy outcomes such as fetal death or congenital monkeypox (5). Clinical investigations have also reported a higher susceptibility to systemic complications in this population, attributed to the immunosuppressed state during pregnancy, which may exacerbate viral replication and disease severity (7). Consequently, early diagnosis through molecular testing and regular monitoring are crucial to manage infection effectively and mitigate risks to both mother and fetus.
The primary method for diagnosing monkeypox is polymerase chain reaction (PCR) testing, which detects the virus’s DNA in lesion samples. PCR is highly specific and can differentiate monkeypox from other orthopoxviruses like smallpox (14). Lesion swabs are commonly used as specimens, and other bodily fluids, such as blood or throat swabs, may be tested depending on clinical presentation. Rapid and accurate diagnosis is essential to guide appropriate isolation measures and clinical management.
Although less commonly used than PCR, serological tests can detect antibodies against the monkeypox virus, indicating past exposure or infection. These tests are more useful for epidemiological studies than acute diagnosis. Antigen detection methods can be an alternative, but they are generally less sensitive than PCR and may not be widely available in all healthcare settings (20).
Since monkeypox can occasionally lead to systemic complications, such as liver or kidney involvement, additional investigations may be warranted, especially in severe cases or high-risk patients. Liver function tests, renal function tests, and complete blood counts can provide insights into the overall impact of the virus on the body and help identify early signs of organ involvement. Imaging studies, such as chest X-rays, may also be necessary if respiratory symptoms are present, as monkeypox can lead to respiratory complications (16,21).
For pregnant patients, additional monitoring is often required to assess fetal well-being, particularly if the mother shows signs of systemic infection. Ultrasound imaging may be employed to monitor fetal growth and detect potential complications, while regular obstetric assessments help track maternal and fetal health. In lactating mothers, investigations should include a thorough assessment to identify any active lesions or symptoms that could pose transmission risks to the infant.
When conducting diagnostic procedures for suspected monkeypox cases, it is important to follow strict infection control measures to prevent transmission to healthcare providers and other patients. Proper personal protective equipment (PPE) and isolation protocols should be implemented to minimize the risk of healthcare-associated infections. These precautions are particularly essential when dealing with pregnant and lactating individuals, as they may have close contact with others, including infants and family members (17).
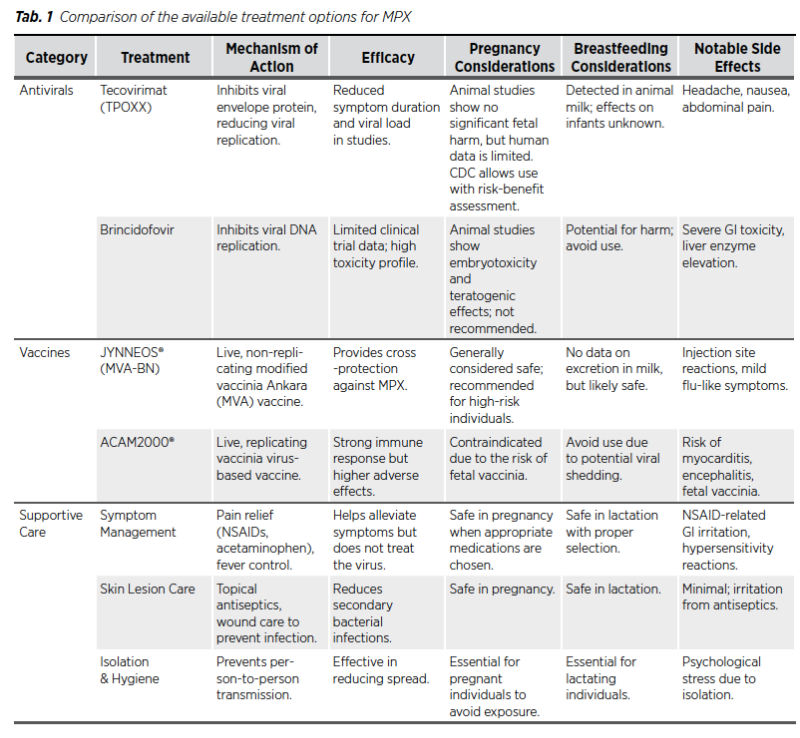
The treatment of monkeypox focuses primarily on supportive care, symptom management, and, in some cases, antiviral therapy. Pregnant and lactating individuals require special consideration to ensure both maternal and fetal safety. Supportive care remains the foundation of treatment, with antivirals such as Tecovirimat considered in severe cases. Alternative therapies should be used cautiously, and any treatment decision must weigh the risks and benefits for the mother and child (14,16).
The current management of monkeypox during pregnancy and lactation focuses on supportive care and antiviral therapies, alongside preventive measures like vaccination for high-risk groups. Tecovirimat, an antiviral approved for smallpox, has shown potential effectiveness against monkeypox and is considered safe for use in pregnant women under medical supervision (6). Animal studies have not demonstrated significant fetal harm when Tecovirimat was administered at doses approximately 23 times higher than the recommended human dosage. However, there is a lack of human data to confirm these findings. The Centres for Disease Control and Prevention (CDC) suggests that Tecovirimat may be considered for treating MPX in pregnant individuals after a thorough discussion of potential risks and benefits (33). Furthermore, animal studies indicate its presence in milk, but the implications for human infants are unclear. Given the potential risk of MPX transmission through close contact during breastfeeding, the CDC advises caution (34). A study by Huggins et al. (2009) assessed tecovirimat’s efficacy in nonhuman primates exposed to lethal doses of monkeypox or variola virus. Treatment with Tecovirimat resulted in a survival rate of 100% in treated animals, compared to 0% in the placebo group (35). The study also noted a significant reduction in lesion formation and viral load in treated subjects (36).
Moreover, animal studies have shown that Brincidofovir can cause fetal harm, including structural malformations and embryotoxicity, even at exposures lower than the expected human dosage. Consequently, its use is not recommended during pregnancy. Women of childbearing potential should use effective contraception during treatment and for at least two months after the final dose. In clinical trials involving immunocompromised patients, Brincidofovir was associated with gastrointestinal adverse events, particularly diarrhoea, which was dose-limiting in some cases (37). Brincidofovir has been studied in clinical trials for other viral infections such as cytomegalovirus (CMV) and adenovirus. Evaluating Brincidofovir for CMV prophylaxis in hematopoietic stem cell transplant recipients, the all-cause mortality at week-24 was 16% in Brincidofovir group compared to 10% in placebo group (37). Therefore, due to the limited data on the safety and efficacy of tecovirimat and Brincidofovir in pregnant and breastfeeding individuals, treatment decisions should involve a careful assessment of potential risks and benefits. Healthcare providers should engage in shared decisionmaking with patients, considering the severity of the MPX infection and the available treatment options (34).
As mentioned above, animal studies provided insights into the efficacy and potential teratogenicity of these antivirals, however; there were limited evidences among the humans, especially concerning pregnant individuals. Due to the current reliance on animal data, should emphasize the need for further research in human populations, particularly during pregnancy.
Vaccination remains a critical preventive measure, particularly with the modified vaccinia Ankara (MVA) vaccine, which has demonstrated efficacy in monkeypox prevention without significant adverse outcomes, making it a viable option for pregnant and lactating women at high risk (5). Supportive treatments to alleviate symptoms and prevent secondary infections, combined with isolation practices, are also essential to minimize the spread and severity of the virus. Nonetheless, further research is needed to confirm the safety profile of these treatments and improve guidelines for managing monkeypox in pregnant and lactating women effectively (7).
For most patients, including pregnant and lactating mothers, supportive care remains the cornerstone of monkeypox treatment. This includes adequate hydration, pain management, antipyretics for fever, and rest to aid the body’s recovery. Skin lesions may require topical treatment to prevent secondary infections, and good hygiene practices are essential to facilitate healing and reduce the risk of bacterial superinfections (4). Managing symptoms effectively can improve quality of life and reduce the likelihood of complications, especially in patients with prolonged or severe symptoms (15).
Antiviral therapiesIn cases where monkeypox poses a high risk of complications, antiviral therapies such as Tecovirimat and Brincidofovir may be considered. Tecovirimat, which targets the viral envelope protein, is an FDA-approved antiviral for smallpox and has shown potential in treating monkeypox. Studies suggest that Tecovirimat may help reduce symptom duration and viral load, though its use in pregnancy and lactation should be carefully evaluated. Brincidofovir, another antiviral that inhibits viral replication, may also be considered in severe cases; however, its safety profile in pregnant and lactating patients remains under investigation (3,9).
Moreover, studies stated that while Tecovirimat and Brincidofovir showed efficacy in animal models, their safety in pregnant humans remained largely unverified. Currently, no randomized controlled trials had been assessed the safety of these antivirals in pregnancy, and their use was also based on extrapolated data from animal studies (38). Given the potential risks, it is crucial that the need for careful consideration before prescribing these treatments to pregnant individuals.
While there is no specific natural remedy for monkeypox, some complementary therapies can support overall health and immunity. Herbal supplements with immune-boosting properties, such as echinacea and elderberry, may offer general support, though they should be used cautiously during pregnancy and lactation and in consultation with a healthcare provider. Topical remedies, like aloe vera or calendula, may provide some relief for skin discomfort, but these should be applied cautiously to avoid irritation or adverse reactions. Since evidence on the efficacy of natural remedies for monkeypox is limited, they should only be considered as adjunct therapies rather than primary treatments (8).
Healthcare providers must carefully assess the risks and benefits of antiviral treatments and medications in pregnant or lactating individuals. Given the potential for teratogenic effects or transmission through breast milk, antiviral therapies should be used cautiously. Supportive care, combined with vigilant monitoring of maternal and fetal health, may be the safest approach for most mild to moderate cases of monkeypox in pregnancy. If antiviral treatment is necessary, close monitoring and informed decision-making with the patient are essential (15,39). Research on emerging therapies for monkeypox is ongoing, with studies examining the safety and efficacy of novel antivirals and immunomodulatory agents. For pregnant and lactating individuals, the development of treatments with lower risk profiles could provide safer options in the future. As new data emerge, treatment protocols will likely evolve to incorporate safer and more effective therapies for high-risk populations, including pregnant and lactating patients (14).
Prevention of monkeypox requires a multi-faceted approach, especially for high-risk groups like pregnant and lactating individuals. Strategies include vaccination, personal protective measures, and public health interventions to reduce exposure. Vaccination can prevent severe disease, while personal protective practices help minimize transmission. Healthcare settings should implement strict infection control protocols, and mothers should take extra precautions to protect their infants (17).
VaccinationVaccination against monkeypox has become a key preventive measure, especially in populations at higher risk. No specific vaccine has been developed to prevent monkeypox virus infection. However, owing to similarity of orthopoxviruses and immunological cross protection smallpox vaccines (vaccinia virus based) were suggested for use in the outbreaks (40).
Currently only two vaccines are in use, JYNNEOS® (live, replication incompetent vaccinia virus) and ACAM2000® (live, replication competent vaccinia virus). JYNNEOS® is a live viral vaccine produced from the modified vaccinia Ankara-Bavarian Nordic (MVA-BN strain) and is an attenuated, non-replicating orthopoxvirus (14).
The MVA-BN vaccine is one vaccine which can be given either before or after exposure to confirmed MPX cases. Pre-exposure prophylactic vaccination protects the people at risk of MPX infections before having had contact with a confirmed case. If the person has been exposed, then the vaccine will reduce the reduce the severity of the impact of the disease. The post exposure vaccination should be done within 14 days of exposure (41). ACAM2000® is not recommended during pregnancy due to the possibility of serious infection in the developing baby called fetal vaccinia. They are also not recommended for those who live in close contact of pregnant women (42). ACAM2000® may also produce a significant cutaneous reaction at the site of inoculation (43).
JYNNEOS™ can be given to women who are either pregnant or periconceptionally. They can be given who are at risk of contact with active cases. Pregnancy alone is not a reason to avoid getting the vaccine if someone is otherwise recommended to receive it (42). The advantage of JYNNEOS® is that it does not cause much of cutaneous reaction at the inoculation site (43). The JYNNEOS vaccine, approved for smallpox and monkeypox, is recommended for individuals at increased risk of exposure, including healthcare workers and close contacts of confirmed cases. For pregnant and lactating individuals, vaccination decisions should be made on a case-by-case basis, considering the potential benefits and risks. Studies on JYNNEOS suggest it may be safe for use in pregnancy, although long-term data are limited. Vaccination can play a critical role in preventing severe disease in exposed individuals (16,20).
Preventive practices such as proper hygiene, avoiding contact with infected individuals, and using protective clothing are essential, especially for pregnant and lactating individuals. Hand hygiene and avoiding direct contact with lesions are simple yet effective methods of minimizing transmission risks. For those living in or traveling to endemic areas, wearing long sleeves, using insect repellents, and staying away from animals that may carry the virus, such as rodents and primates, can reduce the likelihood of exposure (16).
For healthcare providers working with monkeypox patients, including pregnant and lactating healthcare workers, strict infection control measures are essential. This includes using personal protective equipment (PPE), such as gloves, masks, and gowns, to prevent potential exposure. Healthcare facilities must implement protocols to safely manage and isolate suspected cases, reducing the risk of healthcareassociated transmission (17). Pregnant healthcare workers, in particular, should take extra precautions or consider reassignments to lower-risk duties if possible (39).
All individuals who had high-risk sexual behavior are eligible for the vaccine. In addition, the people with exposure to MPX or living with immunocompromised individuals were
recommended to take the vaccines. It was not required for individuals who have been infected by MPX earlier. Regarding the Canadian vaccination guideline, usually, any live vaccine was contraindicated for pregnant ladies. However, if the pregnant women took the vaccine and diagnosed to have pregnancy then there was insufficient data with regards to fetal damage. No adverse effects were seen on developing animal fetus. If the pregnant women was exposed or have immunocompromised status or atopic dermatitis, they were eligible to take the vaccines. This was to ensure benefits exceed risk.
CDC provided the use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses based on recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2022 (44).
Moreover, studies stated that JYNNEOS was recommended for high-risk individuals, but its safety in pregnant individuals was primarily based on animal model data. However, human data on its use during pregnancy remain limited, and ongoing surveillance was needed to establish its safety profile more definitively (45). While JYNNEOS was a non-replicating vaccine and theoretically safer than ACAM2000, more real-world pregnancy-specific data were required before making strong recommendations.
Given the findings, even JYNNEOS was recommended for high-risk individuals, its use during pregnancy remained a topic of ethical debate due to insufficient data from human studies. There may be the ethical dilemma of balancing the potential benefits of vaccination against the unknown risks to both the mother and fetus. Therefore, decision should be made on a case-by-case basis, guided by clinical judgment and available evidence (45).
The CDC recommended people to avoid close, skin-toskin contact with someone who has monkeypox-like rashes, avoid contact with objects and materials that an active patient has used, use of hand sanitizers that contain alcohol, washing hands frequently went a long way to prevent MPX (22). Public health campaigns play a crucial role in raising awareness of monkeypox and educating the public on prevention. Outreach efforts can target highrisk communities, providing information on recognizing symptoms, accessing care, and implementing preventive practices. Early detection and prompt isolation of cases can help limit outbreaks and protect vulnerable populations, including pregnant and lactating individuals. Public health authorities can also provide guidance on vaccination eligibility, especially during outbreaks where risk is elevated (39).
Preventive measures for infants and childrenFor lactating mothers with monkeypox, it is essential to take preventive steps to protect infants from potential transmission. If breastfeeding, alternative methods, such as expressed milk, may be safer if the mother has active lesions. Additionally, mothers should avoid close contact with infants when lesions are present or cover lesions to reduce exposure risk. These precautions can help ensure the safety of infants while allowing them to benefit from maternal care (39). Breastfeeding is often more protective than not breastfeeding. The immunity offered via breast milk confers various antibodies and may contain antibodies against MPX. Moreover, if the mother had active infection or had lesions near areola then breastfeeding or expressing breast milk and feeding was better avoided (32).
Monkeypox poses unique challenges for pregnant and lactating individuals, who may face higher risks of adverse outcomes and transmission concerns due to their close interactions with infants. This review highlights the importance of comprehensive management approaches that address diagnosis, symptom control, and prevention, with a special focus on the needs of these vulnerable groups. Future studies are essential to expand our understanding of the virus’s effects on maternal and infant health and to refine guidelines for safely managing monkeypox in these populations.
Supportive care remains the foundation of treatment, while antiviral therapies offer additional options in severe cases, although their safety profiles in pregnancy and lactation are not fully established. Vaccination plays a vital role in prevention, particularly in healthcare settings and high-risk areas, while adherence to strict infection control measures further protects pregnant and lactating individuals from exposure. Therefore, further research into MPX-specific biomarkers, particularly those indicative of vertical transmission, can improve case management. By establishing the cohort studies and registries for pregnant women with MPX would facilitate monitoring and enhance evidence-based management (7).
Evidence is still limited regarding the exact impact of monkeypox on pregnancy outcomes, lactation, and breastfeeding, underlining the need for more targeted research. Evidence-based clinical recommendations, such as the use of tecovirimat under medical supervision and vaccination in high-risk populations, have been detailed. Additionally, the need for long-term outcome studies has been emphasized to guide future research and interventions. Future studies are essential to expand our understanding of the virus’s effects on maternal and infant health and to refine guidelines for safely managing monkeypox in these populations. Randomized controlled trials should investigate the long-term effects of tecovirimat and the MVA vaccine on maternal and neonatal health and the studies need to focus on viral replication in placental tissues and its impact on fetal development (5,6). Through ongoing research and adaptive healthcare practices, we can improve protection for pregnant and lactating individuals, ensuring safer outcomes for them and their infants amid monkeypox outbreaks.
There were policy gaps in the current MPX response, particularly regarding guidance for pregnant and lactating healthcare workers. Currently, there was a lack of clear and consistent guidance for healthcare workers who were pregnant or lactating, and this gap could lead to uncertainty in managing workplace exposure to MPX. It was recommended that policies be developed to offer protective measures, such as flexible work arrangements, isolation protocols, and additional safety training, to minimize exposure risk while safeguarding maternal and fetal health (46).
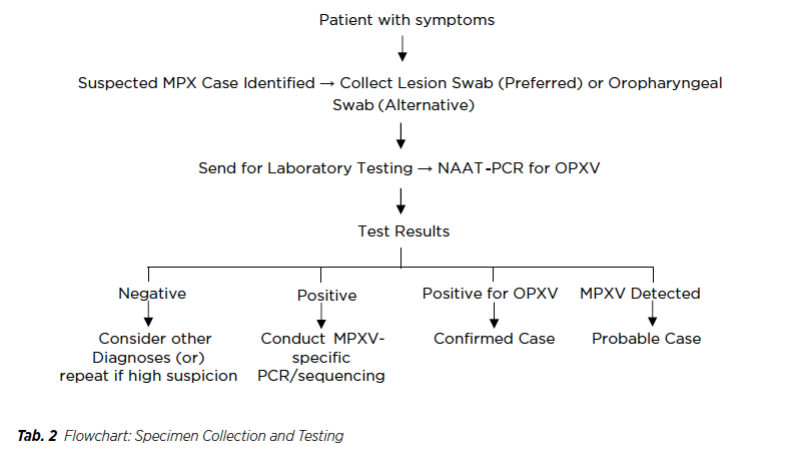
Based on the WHO interim guidance document on monkeypox (MPX) laboratory diagnosis, we have extracted key diagnostic and management pathways into practical algorithms and flowcharts to enhance usability for healthcare providers (47).
Algorithm 1: Diagnosis of Monkeypox (MPX)
1. Identification of Suspected Case
First of all, identification of individual with acute unexplained skin rash, mucosal lesions, or lymphadenopathy and assessment of presence of fever, headache, myalgia, or profound fatigue were necessary. Moreover, healthcare provider needed to ask the history of contact with a confirmed or probable MPX case within 21 days to the patients. Then, exclusion of other common causes of rash (e.g., varicella, syphilis, herpes) might be needed.
2. Specimen Collection
MPX is transmitted primarily via direct contact with infectious lesions, bodily fluids, or contaminated materials. Respiratory droplet transmission is also possible, particularly with prolonged exposure. Therefore, the preferred specimen collection was the lesion swabs (surface, exudate, or crusts) and if no visible lesions, oropharyngeal/anorectal swabs can be done as alternative way.
3. Laboratory Testing
Basically, there were two (2) steps in laboratory testing. In step -1, healthcare workers usually perform nucleic acid amplification test (NAAT) using PCR to detect Orthopoxvirus (OPXV) DNA. Furthermore, in step 2, if positive, healthcare workers needed to conduct MPXspecific PCR or sequencing to confirm clade (Clade I or Clade II).
4. Interpretation of Results
If MPX Detected, it could be Confirmed Case. OPXV detected but no MPX confirmation, it could be Probable Case. If there was a negative test, healthcare provider, should consider to repeat testing if clinical suspicion remains.
Algorithm 2: Management of Monkeypox (MPX)
For confirmed or probable case, the isolation is necessary until all lesions are healed and crusts have fallen off accompanied with the supportive care such as hydration, pain management, fever control. For severe disease, continuous monitoring might be necessary especially for immunocompromised, pregnant, or pediatric patients may require hospitalization.
For severe or high-risk cases, hospitalization is necessary to compensate for dehydration, respiratory distress, secondary bacterial infections, extensive lesions etc. Moreover, antiviral therapy should consider for instance, providing tecovirimat for severe cases.
For contact tracing & post-exposure prophylaxis (PEP): if there were high-risk contacts, healthcare provider should offer post-exposure vaccination within 4 days (up to 14 days if no symptoms) and monitor the contacts for 21 Days for symptoms.
1. Happi C, Adetifa I, Mbala P, Njouom R, Nakoune E, Happi A, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS biology. 2022;20(8):e3001769
2. Isaacs D. Evidence-based neonatal infections: John Wiley & Sons; 2013
3. Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infectious Disease Clinics. 2019;33(4):1027-43
4. Organization WH. Monkeypox and breastfeeding: Key considerations. WHO. 2022
5. Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. The Journal of infectious diseases. 2017;216(7):824-8
1. Monkeypox infection during pregnancy is associated with an increased risk of miscarriage, congenital abnormalities such as microcephaly and stillbirth.
2. Breastfeeding should always be contraindicated for mothers infected with monkeypox, even in the absence of active lesions near the breast.
3. Human-to-human transmission of monkeypox primarily involves respiratory droplets, direct contact with lesions, or bodily fluids.
4. ACAM2000® vaccine is recommended to pregnancy women.
5. The treatment of monkeypox focuses primarily on supportive care, symptom management, and, in some cases, antiviral therapy.
1. True
2. False
3. True
4. False
5. True
There is no funding support for this research.
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
K.M.T, T.M.K and S.D. wrote the manuscript. All authors reviewed the manuscript.
No datasets were generated or analyzed during the current study.
We would like to thank Ms Shazana Mohd for helping us retrieve the full articles using interlibrary facilities.
MPX - Monkeypox MPXV - Monkeypox Virus OPXV - Orthopoxvirus NAAT - Nucleic Acid Amplification Test PCR - Polymerase Chain Reaction