









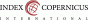

 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Ovarian cancer, which has the highest mortality rate of all gynecologic malignancies, has increased significantly in incidence over the past 50 years. The American Cancer Society estimates that approximately 19,680 women will be diagnosed with ovarian cancer in 2024, and approximately 12,740 women will die from it. The overall 5-year overall survival (OS) rate for ovarian cancer is approximately 30-40%, compared to 93% for localized ovarian cancer and 31% for those with distant metastases. Late diagnosis and resistance to chemotherapy are blamed for the high mortality rate and low OS rate of ovarian cancer (1).
Ovarian cancer exhibits considerable heterogeneity in its molecular, morphological, and histological characteristics. Risk factors for ovarian cancer include age, ethnicity, genetic predisposition, and various lifestyle factors. Early diagnosis is vital for effective treatment of cancer (2). Nonetheless, the absence of clear symptoms often leads to diagnosis at advanced stages. Treatment modalities for ovarian cancer comprise debulking surgery, pharmacotherapy, and radiotherapy. The majority of patients undergo cytoreductive surgery followed by platinum-based chemotherapy (3). Recurrence within six months postplatinum therapy indicates chemotherapy resistance, affecting approximately 70% of patients. Independent predictors of recurrence in ovarian cancer include age, stage, tumor grade, ascites, and surface tumor. Factors such as advanced disease, residual disease volume, neoadjuvant chemotherapy, and BRCA status are associated with disease progression and mortality (4).
Cyclooxygenase-2 (COX-2) is expressed in response to stimuli such as cytokines, mitogens, growth factors, or hormones and is involved in inflammatory and oncogenic processes. COX-2 contributes to tumor development by stimulating angiogenesis, increasing resistance to apoptosis, and causing local immune suppression. Most solid tumors such as lung, liver, pancreas, breast, colorectal, and ovarian cancers, have been found to exhibit COX-2 overexpression. Moreover, patients with tumors overexpressing COX-2 have been shown to have lower response to standard therapy and shorter survival times. Some studies have shown that COX-2 expression in ovarian cancer patients is not associated with histological subtype, ascites, presence of residual disease, or age (5). However, it has been reported that COX-2 expression in patients with epithelial ovarian cancer is associated with age, stage, presence of ascites, and residual tumor status (6). In addition, it has been suggested that COX-2 overexpression may be associated with resistance to chemotherapy in ovarian cancer (7). A meta-analysis found that higher COX-2 expression was associated with poor OS, but not significantly with chemotherapy resistance and DFS (8). It was also determined that COX-2 positivity was significantly associated with various clinical parameters such as age, stage and histology. Another recent meta-analysis showed that patients with higher COX-2 expression had poor OS and lower DFS, and COX-2 expression was associated with FIGO stage, histological type, and age (9). However, more evidence is needed regarding the prognostic value of COX-2 in ovarian cancer. Therefore, this study aimed to determine the factors associated with disease-free survival (DFS) and OS in ovarian cancer patients treated surgically in our clinic and to reveal the relationship of COX-2 positivity with clinicopathological findings.
This retrospective study was conducted at the Department of Obstetrics and Gynecology, Cerrahpasa Medical Faculty, Istanbul University. This study was supported by the Istanbul University Scientific Research Fund (Project No: 1770), and ethical approval was obtained from the Cerrahpasa Medical Faculty Ethics Committee. The tissue blocks and medical data of 74 ovarian cancer patients who underwent surgery between 1995 and 2007 were retrospectively examined.
All patients received primary surgical treatment. Staging was performed according to FIGO classification. Patients with residual tumors of 1 cm or less after surgery or no residual tumor were considered to have optimal surgery, and those with residual tumor tissue of more than 1 cm were considered to have suboptimal surgery. All patients received 6 to 9 courses of platinum-based chemotherapy after surgical treatment. Four patients (5%) received cisplatin (75 mg/m², D1) + cyclophosphamide (1 gr/m², D1) chemotherapy, while the remaining 70 patients (95%) received the combination of carboplatin (AUC-6 (Area Under the Curve), D1) + paclitaxel (175 mg/m², D1). Response to chemotherapy was assessed according to clinical (gynecological) and ultrasound examinations, computer tomography (CT) and magnetic resonance (MR) examinations, and serum CA125 levels. Sensitivity criteria for chemotherapy were determined as the absence of proven disease after first-line treatment and the absence of disease recurrence for 12 months after treatment.
Immunohistochemical staining was conducted in the Pathology Department of Cerrahpaşa Medical Faculty. Two-micron sections from paraffin blocks were mounted on polylysine slides. The slides were placed in an oven overnight at 56°C. Subsequently, they were treated with xylene, absolute alcohol, and 96% alcohol for 15 minutes each. The sections were then washed with distilled water and subjected to EDTA solution in a microwave for antigen retrieval. Following this, hydrogen peroxide (peroxidase block novocastra, Leica Biosystems, USA) was applied for 10 minutes at room temperature. The sections were rinsed with phosphate buffer saline (PBS) and treated with a protein blocking solution for 5 minutes. After another wash in PBS, the sections were incubated with primary antibody COX-2 (Cat no: ab52237, Abcam, USA) for two hours. Following a 30-minute incubation with the secondary antibody, using polylink 2 plus HRP detection kit was applied. After 5 minutes with AEC chromogen, Mayer hematoxylin was utilized for counterstaining. Finally, the tissues were rinsed with water and covered with an aqueous mounting agent for examination.
The examination was performed by a pathologist in a blinded manner (without knowledge of the clinical data of the cases). As a result of the examination, the COX-2 staining rate and the intensity of staining of the tissues were determined. The staining rate was determined as a percentage, and the staining intensity was determined in four degrees as “no staining”: 0, “weak”: 1, “moderate”: 2 and “strong”: 3. Those with a staining rate of 20% or lower in the entire section were considered COX-2 negative, those with a staining rate of 20% and a staining intensity of 1 and above were considered COX-2 positive (Fig. 1) (10).
Statistical analysis utilized SPSS 20 software. The Kolmogorov-Smirnov test assessed data normality. Continuous variables‘ descriptive statistics and categorical variables‘ frequencies were reported. Fisher‘s exact test compared categorical data, while independent samples t-test and Mann-Whitney U test analyzed continuous data. Kaplan-Meier and Cox regression analyses evaluated disease-free survival (DFS) and overall survival (OS) factors. A p-value threshold of <0.05 indicated statistical significance.
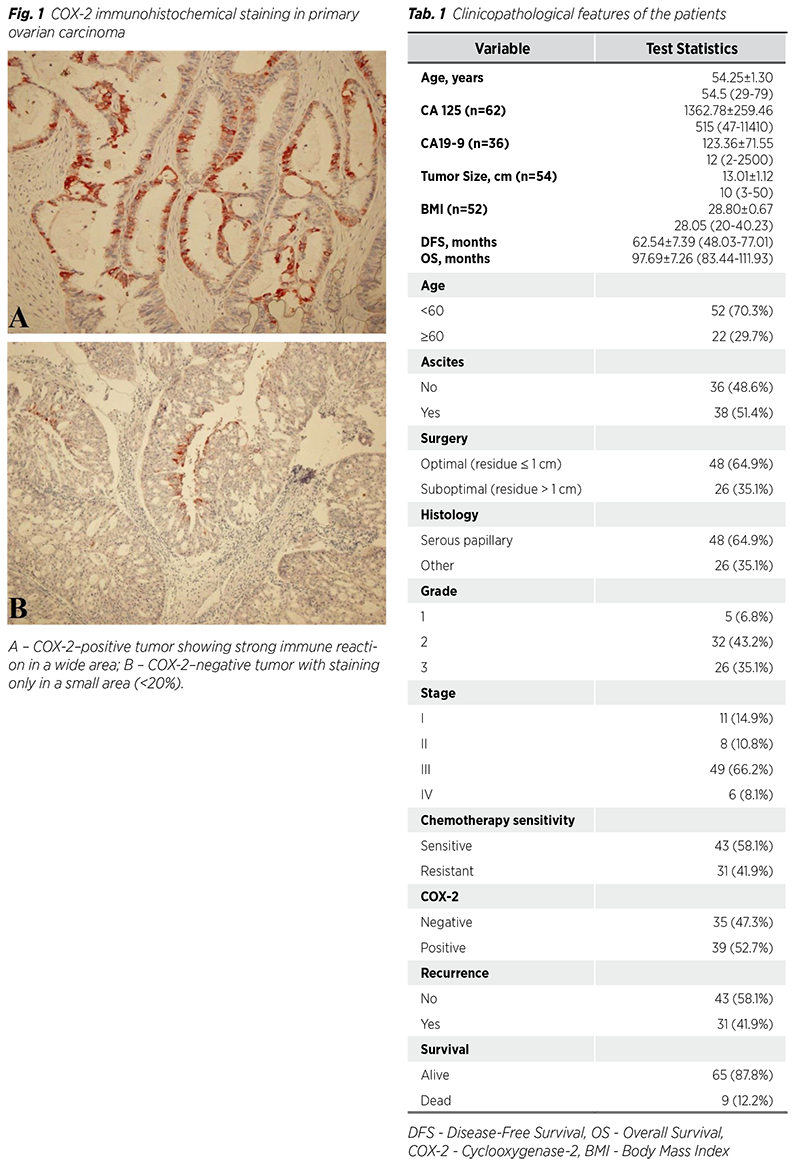
The clinicopathological characteristics of the patients are given in Tab. 1. The mean age of the patients was 54.25 ± 1.30 years, and 22 (29.7%) patients were ≥60 years old. The mean tumor size was 13.01 ± 1.12 cm, the mean CA 125 value was 1362.78 ± 259.46, the mean CA 199 value was 123.36 ± 71.55, the mean BMI was 28.80 ± 0.67 kg/m2, the mean DFS was 62.54 ± 7.39 months, and the mean OS was 97.69 ± 7.26 months. Thirty-eight (51.4%) patients had ascites and 26 (35.1%) patients had postoperative residual volume >1 cm. Forty-eight (64.9%) patients had serous papillary cancer, 32 (43.2%) patients had grade 2, and 49 (66.2%) patients had stage III. Forty-three (58.1%) patients were sensitive to chemotherapy, and 39 (52.7%) patients were COX-2 positive. Recurrence was observed in 31 (41.9%) patients, and 9 (12.2%) patients died during the study period.
Comparison of clinicopathological data according to COX-2 positivity is given in Tab. 2. CA125 level, tumor size, number of patients with ascites, number of patients with residual >1 cm, and number of stage III patients were numerically higher in COX-2 positive ovarian cancer patients than in COX-2 negative ovarian cancer patients. However, these differences were not statistically significant because the sample size was small. However, COX-2 positivity was significantly higher in Stage III-IV ovarian cancer than in Stage I-II ovarian cancer (p = 0.032). Recurrence and survival were not significantly associated with COX-2 positivity.
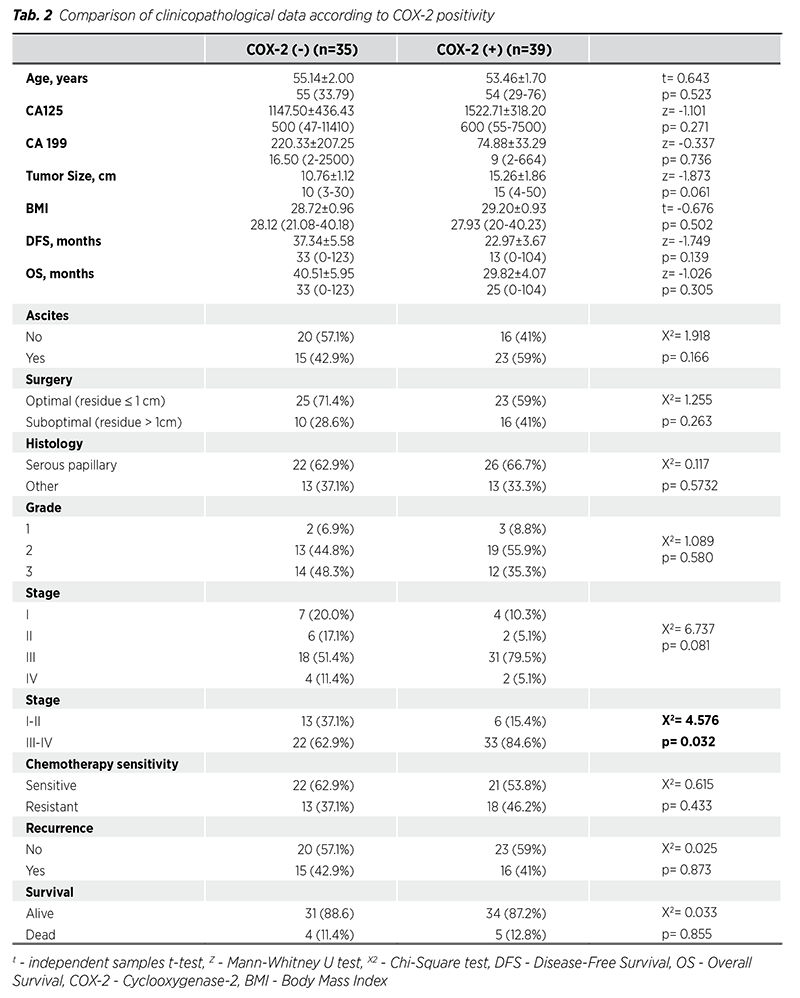
Kaplan-Meier analysis results of clinicopathological data related to OS are presented in Tab. 3. OS of patients with postoperative residual volume >1 cm was significantly lower than OS of patients with postoperative residual volume <1 cm (p < 0.001), and OS of patients resistant to chemotherapy was significantly lower than OS of patients sensitive to chemotherapy (p = 0.001). In addition, OS of stage III-IV patients was significantly lower than OS of stage I-II patients (p = 0.056). Age, presence of ascites, histological subtype, grade, COX-2 positivity, and recurrence did not affect OS.
Cox regression analysis results of the relationship between clinicopathological data and OS and DFS are given in Tab. 4-5. Among clinicopathological data, only resistance to chemotherapy was associated with OS (HR = 12.50, p = 0.005). Age (HR = 2.23, p = 0.030), histological subtype (other histological subtypes HR = 0.36, p = 0.019), stage (HR = 3.64, p = 0.037 for stage III; HR = 8.06, p = 0.013 for stage IV), and chemotherapy sensitivity (HR = 5.46, p < 0.001 for chemotherapy resistance) were associated with DFS.
This retrospective study focused on clinicopathological findings related to DFS and OS in patients with ovarian cancer and the relationship of COX-2 expression with these findings. Age, chemotherapy resistance, histological subtype, and stage were determined as predictors for DFS, while chemotherapy resistance was determined as a predictor for OS. COX-2 positivity was higher in stage III-IV patients than in stage I-II patients. However, COX-2 positivity was not associated with DFS or OS.
Ovarian cancer, which has a high mortality rate, is the most common cause of death from gynecological tumors. Since it does not have specific symptoms, the early diagnosis rate is low, and 70% of patients present with advanced stage disease (11). Due to both advanced stage and chemotherapy resistance, the 5-year OS rate in advanced stage ovarian cancer is approximately 29.2% (12).
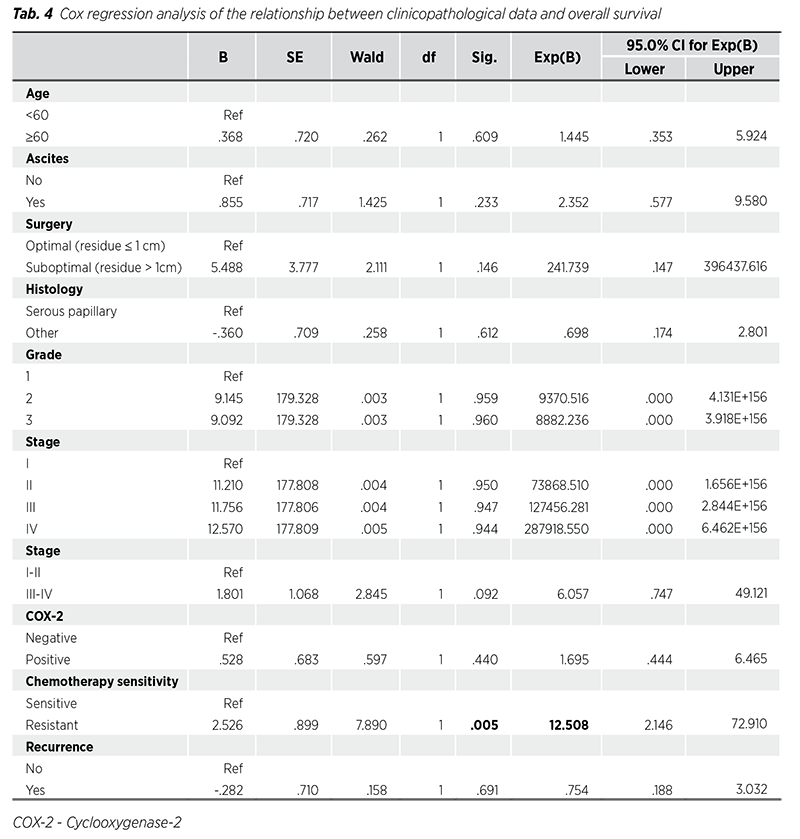
In addition, the recurrence rate within 18 months in women with advanced stage ovarian cancer is 70 - 90% (13). Due to the high mortality and recurrence rates, it is important to determine predictors of OS and DFS in ovarian cancer. Upadhyay et al. (14) determined the 5-year DFS and OS of patients with stage I-II ovarian cancer as 76.9% - 55.9% and 89.4% - 78%, respectively. In their study, they found that age, stage, and grade were associated with recurrence in univariate analysis, and only tumor grade was associated with DFS and OS in multivariate analysis. Hsieh et al. (15) showed that FIGO stage, histologic type, and tumor grade were significant prognostic factors for 5-year DFS in a Cox regression model. The researchers found that FIGO stage was the only factor associated with 5-year OS. Other studies have noted that advanced disease, excess residual disease volume after surgery, BRCA wild-type diseases, and neoadjuvant chemotherapy were associated with worse OS (16). In another study, it was determined that age and FIGO stage predicted DFS, while menopausal status predicted OS (17). A recent study determined that age, clinical stage, histological subtype, tumor size, and mutation number were associated with DFS and OS (18). Similar to previous studies, in our study, age, stage, histological subtype, and chemotherapy resistance were determined to be predictors for DFS, and chemotherapy resistance for OS. Because the mortality rate in our study was low, the relationship between other clinicopathological data and OS could not be determined at a significant level.
COX-2, the enzyme involved in the conversion of arachidonic acid to various prostaglandins, has been shown to be overexpressed in inflammation and many malignancies.
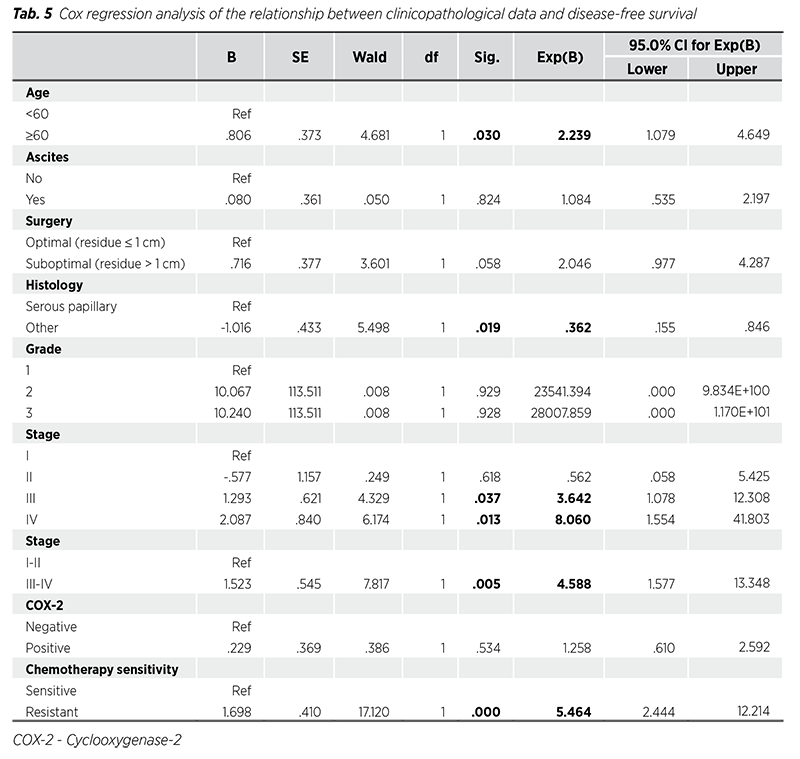
Moreover, COX-2 expression has been reported to have a prognostic effect in many tumor tissues (19). COX-2 has been shown to be an important factor in tumor invasion and metastasis in ovarian cancer. Ferrandina et al. (20) suggested that upregulation of COX-2 expression in ovarian cancer cells is an important factor in cancer development. Raspollini et al. (21) demonstrated that COX-2 positivity is associated with chemotherapy resistance and recurrence. In ovarian cancer cells, it was shown that COX-2 can reduce sensitivity to cisplatin and increase cisplatin resistance. Therefore, it is suggested that COX-2 may be a molecular marker to predict chemotherapy resistance in ovarian cancer. In addition, in recent studies, it has been documented that the selective COX-2 inhibitor celecoxib has synergistic anticancer effects when combined with chemotherapy drugs (22). Gómez-Valenzuela et al. (23) have shown that high COX-2 expression is linked to cell dysfunction and lower effector activity of natural killer cells, changes in the immune ecosystem, and poor survival. The researchers suggested that first targeting COX-2 could be useful in improving the effectiveness of immunotherapy for ovarian cancer patients. Upregulation of VEGF-C is induced by the COX-2 enzyme, and there is a strong correlation between COX-2 and VEGF-C (24). Bhaskari et al. (25) reported in their study that Ki-67, tissue COX-2 and VEGF-C plasma levels were strong and independent predictors of poor prognosis and that tissue COX-2 and VEGF-C levels strongly predicted recurrence. In a meta-analysis of 17 studies by Lee et al. (8), higher COX-2 expression was documented to significantly predict poor OS. Moreover, when studies were included that adjusted for stage, histology, and age, a more pronounced association between COX-2 expression and poor OS was found. While there was a significant association between COX-2 positivity and clinical parameters such as age, stage, and histology, higher COX-2 expression was not significantly associated with poor DFS and chemotherapy resistance. A meta-analysis evaluating 18 studies including 1,867 ovarian patients determined that higher COX-2 expression was associated with poor prognosis for ovarian cancer patients. Researchers reported a correlation between COX-2 expression and FIGO stage, histological type, and age of patients (9). The results of the same meta-analysis revealed that patients with higher COX-2 expression had lower DFS and OS. In our study, we evaluated the relationship between COX-2 positivity and clinicopathological findings. We determined that there is a relationship between COX-2 positivity and advanced stage ovarian cancer. Additionally, although CA125 level, tumor size, number of patients with ascites, number of patients with postoperative residual volume >1 cm, and number of patients with chemotherapy resistance were numerically higher in COX-2 positive patients than in COX-2 negative patients, these differences were not statistically significant. In our study, DFS and OS durations of COX-2 positive patients were numerically lower than in COX-2 negative patients, but they were not statistically significant. The reason why statistical significance was not reached is probably due to our low number of patients.
There are several limitations to our study. The study‘s first drawback is the heterogeneous population and its retrospective nature. Due to the retrospective nature of the study, it is prone to selection bias and confounding factors, which may affect the validity of the findings. Additionally, results could have been impacted by modifications in surgical methods during the course of the 6-year study. The study‘s modest sample size and single-center design constitute its second drawback. Since the study is singlecenter, the results cannot be generalized. Another limitation is the exclusion of patients undergoing laparotomy procedures. This may lead to a selection bias as different baseline characteristics of these patients may affect the results. Another limitation was the exclusion of patients who could not be reached by phone and incomplete records found in the files scanned in the hospital record system. Therefore, greater sample sizes and multicenter, randomized controlled investigations should validate the findings of this investigation.
Our study findings revealed that age, chemotherapy resistance, histological subtype, and stage were predictors for DFS, and chemotherapy resistance was predictor for OS. We also determined that COX-2 positivity is associated with cancer stage and numerically reduces DFS and OS. Determining the relationship between chemotherapy resistance and COX-2 levels in advanced stage patients is important for the treatment strategy. Therefore, larger sample size studies including molecular studies containing genetic profiling are needed to better understand the role of COX-2 in ovarian cancer heterogeneity.
Acknowledgements: We would like to acknowledge the www.makaletercume.com for their outstanding scientific proofreading and editing services that was provided for this manuscript.
Author contributions: Conceptualization: [AI]; Acquisition of data: [AI], [RS], [TB]; Analysis and/or interpretation of data: [AI], [TB]; Drafting the manuscript: [AI], [RS], [TB]; Revising the manuscript critically for important intellectual content: [AI], [RS], [TB]; Approval of the version of the manuscript to be published: [AI], [RS], [TB].
Conflict of Interest statement: The authors have no conflict of interest in this study.
Funding: This study was supported by the Istanbul University Scientific Research Fund (Project No: 1770).
Ethical statement: Ethical approval was obtained from the Cerrahpasa Medical Faculty Ethics Committee.
Data availability statements: The data that support the findings of this study are available from the corresponding author upon reasonable request.