










 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.

Aim: In this study, we aimed to analyze the hyperprolactinemia levels and to determine the correlation of symptoms with MRI images and to optimize their health care spending.
Methods: A total of 363 patients were included in this retrospective study, which was conducted between 01.01.2019 and 01.01.2021. Hyperprolactinemia is defined as a serum prolactin level of > 33.5 ng/mL in women. Patients with normal TSH values and isolated hyperprolactinemia were included in the study. The patients were examined in 3 groups, with prolactin levels between 33.5-70 ng/mL (first group), 70-99 ng/mL (second group), and prolactin levels 100 ng/mL and above (third group). The MRI results, treatment modality, treatment outcome and the MRI results of these patients were evaluated.
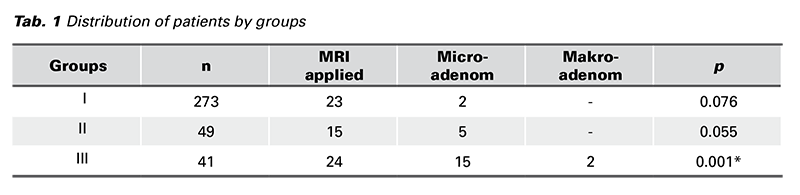
Results: The number of patients included in the first group was 273/363 (75.2%), the number of patients undergoing MRI was 23/273 (8.4%), and the number of patients in whom microadenoma was determined was 2/23 (8.7%). In the 2nd group were 49/363 patients (14%), MRI had 15/273 (30%), and microadenoma 5/15 (33%). In the 3rd group were 41/363 patients (11%), MRI had 24/273 (58%), and microadenoma 15/24 (33%). Macroadenoma was determined in the 3rd group only. MRI was not cost-effective in the first group; but this group benefited most from medical treatment. Patients in the 2nd and 3rd groups were more likely to undergo detection of microadenoma on MRI and the rate of surgical treatment was higher in 3rd group. There was a statistically significant difference between groups 1 and 3 in terms of detecting microadenoma on the MRI (p < 0.001). The prolactin levels of patients with microadenoma on MRI were statistically higher than in patients with normal MRI (p = 0.003).
Conclusion: It would be more appropriate to request MRI for patients with high (> 100 ng/mL) prolactin levels. Requesting tests according to the prolactin levels of the patients will reduce the health cost.
Patients with hyperprolactinemia present to the hospital with the complaints of galactorrhea, irregular menstruation, infertility, and sexual dysfunction (1). With the onset of menopause, the stimulating effect of estrogen on prolactin disappears and circulating prolactin levels decrease (2). Neurological and ophthalmological manifestations are also common, mainly presenting with headaches and changes in the visual fields. Therefore, early diagnosis becomes necessary to select the appropriate treatment (3). Prolactinomas or pituitary adenomas are classified as micro (< 10 mm) and macro-adenoma (> 10 mm) (4). The treatment options include surgery, radiotherapy and dopaminergic agonists (cabergoline, bromocriptine, and quinagolide), which are considered first-line treatments. Cabergoline and bromocriptine are the most commonly used drugs worldwide. These drugs normalize the PRL levels, restore gonadal function and significantly reduce the tumor volume of prolactinomas (5). Surgical treatment is recommended for patients in whom medical treatment has failed or for patients with vision loss or acute tumor complications (6). Sometimes, the biologically inactive prolactin-IgG complex is cleared more slowly than the monomeric prolactin, leading to “pseudo-hyperprolactinemia” (7). True hyperprolactinemia caused by biologically active prolactin is associated with suppression of gonadotropin secretion and gonadal activity (8). This study aimed to investigate the relationship between prolactin levels and clinical manifestation and to determine the correlation of with MRI finding, and to optimize visits to the specialist and define the costeffectiveness of medical imaging.
This study was designed retrospectively and was conducted among patients who presented to the Koru Hospital Ankara Obstetrics and Gynecology outpatient clinic between 01.01.2019 and 01.01.2021. Patients whose prolactin level was requested on the second-fifth day of menstruation with any complaint and in whom the result was higher than the cutoff value were included in the study. The data of 449 patients with high total prolactin levels were obtained. The complaints were reviewed. However, only 363 female patients with isolated hyperprolactinemia between the ages of 18-45 were included in the study. The patients with normal TSH values, those without breastfeeding and without an accompanying endocrine disease were included to the study. The information about the patients was obtained from the database of the hospital. The criteria for exclusion from the study were: hyperprolactinemia with high TSH levels or other endocrine diseases, breast-feeding, breast cancer, cerebrovascular event, chronic renal failure, use of antidepressants or antipsychotics, thoracic surgery or trauma, liver cirrhosis, polycystic ovary, and taking a blood test, except for 2-5 days of menstruation. The parameters examined in the study were: prolactin levels, patients’ complaints, age, number of parities, MR imaging, treatment modalities and the treatment outcome. The patients were divided into 3 groups according to their prolactin level. The first group included patients with prolactin levels between 33.5-70 ng/mL, group 2 between 70-99 ng/mL, and group 3 patients with prolactin levels of 100 ng/mL and above. The complaints and the MR imaging rates were analyzed in each group.
Within the scope of the study, the data of 449 patients were retrospectively reviewed and 363 patients who fulfilled the inclusion criteria were included in the study. The mean age of the patients was 31.9 ± 7.2 (median 31, range: 16-46) years. Of the patients included in the study, 267 had no previous anamnesis of pregnancy, while 51 had one, 31 had two, 8 had three, and 3 had four pregnancies.
The median prolactin level of the patients was 50 (mean was 65.4 ± 41.6, range: 33.75-323) ng/mL. No correlation was observed between the prolactin level and the patient age (r = 0.85, p = 0.054). However, there was a very weak and positive correlation between the prolactin level and parity (r = 0.131, p = 0.007). On the other hand, according to the regression analysis, these two factors were not causally related to each other.
Of the patients included in the study, 279 had presented with menstrual irregularity, 6 with only galactorrhea, 3 with galactorrhea plus headache and 73 with the desire to have a child. When evaluated based on complaints, no statistically significant difference was found between the complaints and the prolactin levels.
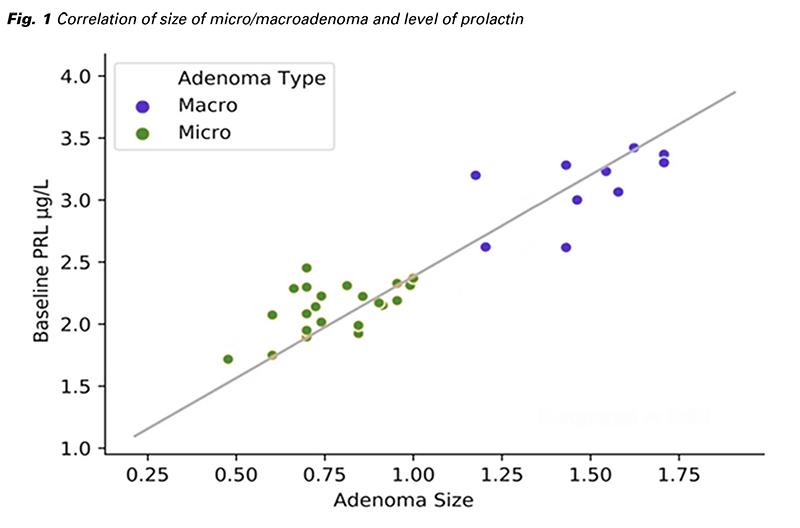
When evaluated according to the MRI results, although the prolactin levels of patients with microadenoma on MRI were statistically higher than patients with normal MRI (p = 0.003), there was no significant difference compared to patients with Rathe cyst on MRI (p = 0.275). In addition, there was no statistically significant difference between the prolactin levels in patients with normal MRI findings (p = 0.871).
The patients were divided into 3 groups according to their prolactin levels. The first group consisted of patients with a prolactin level up to 70 ng/mL, group 2 patients with a prolactin level between 70-99 ng/mL, and group 3 patients with a prolactin level of 100 ng/mL and above. There were 273 patients (75%) in group 1, 49 patients (14%) in group 2 and 41 patients (11%) in group 3. MRI was performed in 23 (8.4%) patients in group 1, 15 (30%) patients in group 2, and 24 (58.5%) patients in group 3. Microadenomas were detected in 2 (8.7%) patients in group 1, 5 (33%) in group 2, and 15 (62.5%) in group 3.
Macroadenoma was detected in only 3% of all patients. All of these patients were in group 3. There was no statistically significant difference between the 2nd and 3rd groups in terms of detecting microadenoma on MRI (p = 0.076). There was no statistically significant difference between groups 1 and 2 in terms of detecting microadenoma on MRI (p = 0.055). However, there was a statistically significant difference between groups 1 and 3 (p < 0.001) (Tab. 1) Spontaneous pregnancy after medical treatment had developed in 14 (26.4%) of 53 patients who had presented with the desire to have a child in group 1, 6 (66.6%) of 9 patients in group 2, and one (11%) of 9 patients in group 3. Surgical treatment was applied to 8 (19.5% patients) in group 3 and only 1 (2%) patient in group 2. When the pregnancy rates were compared, although there was a statistically significant difference between the 1st and 2nd groups, and between the 2nd and 3rd groups (p = 0.016 and p = 0.015, respectively), there was no statistically significant difference between the 1st and the 3rd groups (p = 0.321).
Cost effectiveness was calculated with this formula:
Cost new strategy - Cost current practice > 0
Eff new strategy - Eff current practise
MRI was performed in 23 (8.4%) of 273 (75%) patients included in the first group, and only 2 (8.7%) microadenomas were detected. Macroadenoma was not detected in this group. This indicates that the probability of detecting macro and microadenoma in patients with a prolactin value of 70 and below is low and below this value. The costly MR imaging method shows that it is not cost-effective in terms of health expenditures (cost-effectivite ratio 0.9618).
In the second and third groups, the number of patients, the number of MRIs requested and the number of microadenomas detected were in the order of: n = 49 (14%), n = 15 (30%), n = 5 (33%); n = 41 (11%), n = 24 (58%), n = 15 (62%). No macroadenoma was detected in the second group, and n = 2 (8.3%) macroadenomas were found only in the third group. Magnetic imaging should be requested with a serum prolactin level of 100 and above (cost-effectivite ratio 0.8263). Imaging methods help physicians in the diagnosis and treatment. However, it should be considered whether or not each imaging procedure changes the treatment decision, improves patient outcomes, and affects the costs (9). There was a statistically significant difference between groups 1 and 3 in terms of detecting microadenoma on MRI (p < 0.001).
The cut-off value for macroprolactinemia was determined differently in different studies (10-11). We found the rate of our patients diagnosed with macroprolactin as 3%, and all of these patients were in group 3. As a matter of fact, this value was found to be between 8-66% in different studies (12- 13). In the 3rd group, both the rate of MRI imaging and the rate of microadenoma in MRIs were higher than the other groups. In similar studies, this rate was found to be 21.1% (14). The need for surgical treatment in group 3 was n = 8 (19.5%) and only n = 1 (2%) in group 2. This indicates the importance for using imaging methods for the success of the treatment of patients with a serum prolactin value of > 100 ng/mL. As seen in our study, we think that it would be more appropriate to request MRI for patients with high prolactin levels. In our study, we found that requesting it in groups 2 and 3 was more accurate in terms of detecting microadenoma. We observed that 279 patients with high prolactin levels presented to the clinic due to irregular menstruation or secondary amenorrhea. This was found to be 78% of the patients with high prolactin levels. The rate in similar studies varies between 39-59% (14-15). Medical treatment was begun in this group of patients and regular cycles were achieved with a rate of 91% after the treatment. In our study, the rate of patients who presented with the desire to have child was 20%. Spontaneous pregnancy developed in 26.4% of our patients included in this group, an average of 2.2 months after medical treatment had begun. Cabergoline was used in all medical treatments and the patient group that benefited most from medical treatment was group 1. Hyperprolactinemia affects the hypothalamic-pituitary-gonadal axis and reduces ovulation, resulting in infertility. In some studies, the infertility rates due to hyperprolactinemia were found to be 40% (16).
The prolactin value was found to be above 100 in 77% of the patients who presented with the complaint of galactorrhea. The highest prolactin level was observed in patients with galactorrhea. Microadenoma was detected in 66% of the patients who presented with the complaint of galactorrhea and macroadenoma in 11%. Some authors also stated that MRI should be requested from patients with high prolactin levels (17). Therefore, we believe that it would be better to request MR imaging in patients presenting with this complaint in terms of detecting micro/macro adenoma. There was a correlation between prolactin levels and MRI findings (microadenoma/macroadenoma) in patients in group 3, and hence, clinicians should definitely perform pituitary imaging in the presence of high prolactin values). In our study, no relationship was determined between MRI determination of Microor macro-adenoma and the patients’ complaints. However, despite the known fact that there is a positive relationship between visual disorder and headache (18), there was no patient presenting with the complaint of visual disorder in our study. The reason for this is that the patients were selected from the Obstetrics/Gynecology Polyclinic, and that patients with the complaints of visual disorder and headache had presented to the Neurology and Ophthalmology departments.
In conclusion, in patients with high (100 and above) prolactin levels, the probability of detecting micro/ macro adenoma on MR imaging is high. When the serum prolactin level is under 70 ng/mL, the success rate of medical treatment is high and requesting MRI for patients with prolactin value of < 70 ng/mL does not affect the diagnosis and treatment of patients and does not provide benefit. This approach will help reduce unnecessary expenditures on health care in primary care centers.