Impact of Obesity and Weight Management on Women with Polycystic Ovary Syndrome and Coexisting Obesity – A Brief Narrative Review
How to cite this article: Ghafoor S. Impact of Obesity and Weight Management on Women with Polycystic Ovary Syndrome and Coexisting Obesity – A Brief Narrative Review. Actual Gyn. 2023;15:21-31
Obesity, an ongoing pandemic, is associated with obesity-related androgenic, reproductive, and metabolic comorbidities in females. Polycystic Ovary Syndrome (PCOS) is a multifaceted endocrinopathy that commonly manifests with hyperandrogenic, reproductive, and metabolic dysfunctional features. Obesity and PCOS often coexist. Insulin resistance and subsequent hyperinsulinemia are key factors implicated in the clinicopathological manifestations of PCOS and associated metabolic syndrome. Obesity may amplify these effects, thus, affecting adolescent girls and women of childbearing age. Evidence supports weight loss in achieving favourable endocrine, metabolic, and reproductive outcomes in women with Polycystic Ovary Syndrome and coexisting obesity. Therefore, an effective weight loss strategy should be considered as a front-line intervention in this patient population, with emphasis on fertility timeline-related management in reproductive-aged women, where applicable. This brief narrative review provides insight into the impact of obesity and weight loss on women with Polycystic Ovary Syndrome and coexisting obesity.
Key words: PCOS, Obesity, Overweight, Weight Loss, Polycystic Ovary Syndrome, Exercise, Lifestyle intervention, Bariatric surgery
Introduction
Polycystic Ovary Syndrome (PCOS) is an endocrine disorder with a prevalence of 5% to 15% (1). The etiopathology of this perplexing endocrinopathy is still not completely understood; however, the complex interplay of genetic and environmental traits may play some role (1,2). Due to the heterogeneous nature of the disease, women may experience a spectrum of clinical manifestations of variable intensity from adolescence to menopause (2,3).
Various mechanisms are hypothesized and proposed for the etiopathogenesis of PCOS. This multifaceted syndrome has reproductive, metabolic, and psychological dysfunctional features (summarized in Fig. 1) (4). Numerous evidence-based guidelines mention that PCOS diagnostic criteria require at least two of the following: ovulatory dysfunction, hyperandrogenism, and polycystic ovaries on ultrasound-based imaging (5). Its clinical reproductive features include menstrual irregularity, hirsutism, anovulation-induced infertility, and risk of pregnancy-related complications (6,7). In PCOS, LH/FSH ratio is often altered due to disturbance in the gonadotrophin axis (8). PCOS-related metabolic disturbances increase the individual’s risk of prediabetes, diabetes mellitus, cardiovascular disease, dyslipidemia, and metabolic syndrome (2,9). Emerging evidence indicates an association of PCOS with psychological features such as mood disorders, anxiety, and depression (10-12). Women with PCOS tend to have a poor mental quality of life (13). Various treatment options are available to address diverse symptoms of PCOS, but none of the therapies has been proven yet to combat and cure PCOS (14).
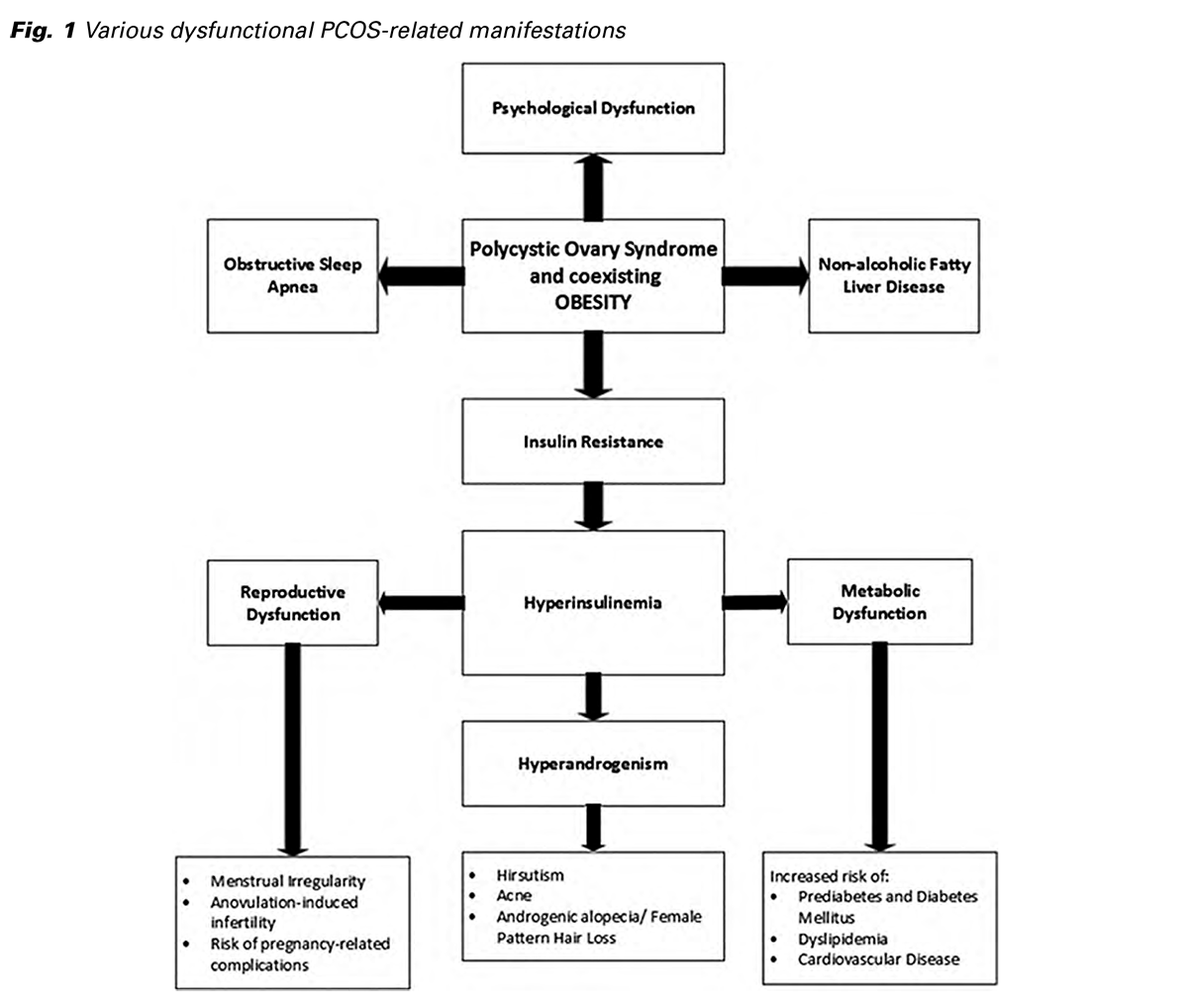
Obesity is prevalent in around 50-70% of women with polycystic ovary syndrome (15,16). Despite the availability of diverse published data, the exact etiopathogenesis of obesity is not clearly explained in the patient population affected with PCOS (16). There is research evidence suggesting androgen excess in women with PCOS, which favours abdominal fat deposition and induces obesity (2). This contributes to insulin resistance and hyperinsulinemia, thus facilitating more androgen secretion. The aforementioned vicious pathological phenomenon and hypothalamic- pituitary-ovary axis dysfunction often result in anovulation and subsequent infertility. It is noted that obese women with PCOS may enhance their ovulation rates through effective weight reduction (16).
Limited evidence is available concerning the progression of PCOS-linked comorbidities over the women’s reproductive lifetime. Research shows that the risk of PCOS-related comorbidities such as dyslipidemia, cardiovascular disease, hypertension, and diabetes mellitus is amplified in the presence of coexisting obesity (4). Although PCOS is considered an obesity-related disorder, the correlation between the two conditions may not imply causation despite the increased prevalence of PCOS in obese women (17). More methodological research and RCTs are required for this patient population to define PCOS-specific weight loss stratagems and therapeutics regimens with long-term favourable clinical outcomes.
Aims and objective
Due to a lack of robust evidence-based research, the exact impact of weight loss remains unanswered for overweight/obese PCOS women in their reproductive years who are chronically struggling with reproductive, metabolic, and endocrine abnormalities. Therefore, this narrative review is sought to assess mainly two objectives: first, to assess the negative health impacts of obesity in reproductive-aged women with PCOS, and second, to review various effective weight management stratagems and related outcomes in this patient population.
Materials and methods
The current narrative review provides insight into the prolific medical literature on the impact of obesity and weight loss on women with PCOS and coexisting obesity. For this purpose, an electronic PubMed database search is performed to identify the relevant research articles. Reference lists of selected research papers are also checked and reviewed to identify the relevant studies and expand this search.
This narrative review includes much of the scholarly work published from 1992 to 2022 and is thus stretched to the last three decades. Preference was given more to recently published literature with relevance to the area of interest. One hundred scientific publications are finally identified and included in this review. These publications are selected by searching the PubMed database while using the combination of the following keywords: “polycystic ovary syndrome,” “PCOS,” “overweight,” “obesity,” “weight loss,” “exercise,” “lifestyle intervention,” and “bariatric surgery.”
Publications evaluating the pediatric population are excluded as those are beyond the scope of this review. Duplicate articles and irrelevant papers are also excluded from the data searched via the PubMed database.
Discussion
Obesity and PCOS often coexist (2,4). PCOS is more prevalent in obese women than those with normal body weight (18,19). Research indicates a six times higher obesity risk in adolescents with polycystic ovary syndrome compared with those without PCOS (20).
The complex pathogenesis of PCOS is often accentuated in obese women (4,21). The intricate interplay of hormonal, genetic, and cardiometabolic features in PCOS with concurrent obesity makes this condition challenging (6). Insulin resistance and excessive adiposity are firmly associated with exacerbation of the clinical severity of PCOS, including anovulation and subfertility. Recent research suggests a possible link between obesity, glucagon-like peptide-1 kinetics alteration, and pathogenesis of PCOS (22). Various diverse research studies reflect on how excess weight gain plays a mediating role in underlying mechanisms implicating PCOS (6,23). The impact of obesity and various weight management strategies on women affected with PCOS and coexisting obesity is briefly discussed in the succeeding sections of this narrative review.
A. The impact of obesity on women with Polycystic Ovary Syndrome
PCOS affects up to 1 in 10 women globally (24). Based on body mass index criteria, at least 50% of PCOS patients are reported to be overweight or obese (15,25). Obesity contributes to the increasing prevalence of PCOS (18). Research-based evidence shows a causal and genetic link between obesity and polycystic ovary syndrome (4). Also, obesity enhances the expression of the PCOS phenotype, particularly in women genetically predisposed to PCOS (4). A research study based on data from the Northern Finland Birth Cohort 1966 suggests a correlation between obesity in adolescence and adulthood and self-reported PCOS-related symptoms in women aged 31 years (26).
The majority of PCOS patients (between 50-90%) are found with insulin resistance, although the underlying mechanisms implicated in insulin resistance and subsequent hyperinsulinemia are poorly understood (27,28). In PCOS, hyperinsulinemia (secondary to insulin resistance) leads to enhanced steroidogenic effect, hyperandrogenemia, ovulatory dysfunction, and dysmetabolic features (4,6,29).
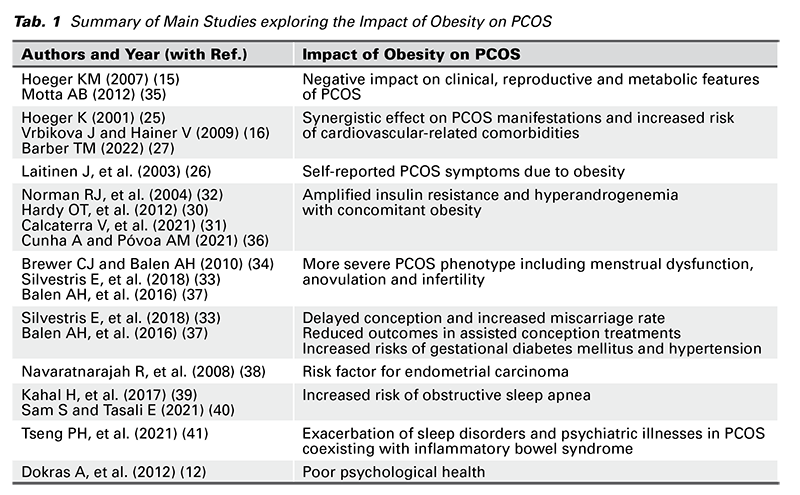
Adiposity accentuates insulin resistance (30). In PCOS, excess androgen levels promote insulin resistance and hyperinsulinemia, which further worsens hyperandrogenism and enhances LH secretion, thus establishing a vicious cycle (31). In short, both obesity and PCOS synergistically contribute to insulin resistance and high serum insulin levels, thus, intensifying the hyperandrogenic state (30,32). Successful weight loss strategies in obese women with concurrent PCOS may improve metabolic, androgenic, and biochemical parameters and clinical outcomes such as restoration of ovulation and menstrual cyclicity and increased chances of conception (6,33,34). Literature on various impacts of obesity on PCOS is briefly summarized in Table 1.
PCOS accounts for 70-80% of anovulatory infertility (36,37). Apart from infertility, other common distressing symptoms of PCOS include menstrual irregularity, hirsutism, and psychological impacts such as anxiety, depression, and low self-esteem (29,42). Obesity is an independent risk factor for subfertility in obese females (without PCOS) (33,34). It is associated with diminished reproductive outcomes in women with spontaneous conception and those seeking fertility treatments such as ovulation induction and IVF (33,34). The link between excessive body weight and poor fertility outcomes is indisputable (22). Research has shown that weight loss (10% of the body weight) may help improve the pregnancy and live birth rates in overweight women (43).
Multiple studies suggest the association of PCOS with an increased risk of endometrial carcinoma (3,44,45). Other noteworthy risk factors for this malignancy include obesity, insulin resistance, hyperinsulinemia, and hyperandrogenism (38,46,47).
PCOS patients are at risk of developing a sleep disorder called obstructive sleep apnea (OSA), which is characterized by periodic partial or complete obstruction of the upper airway leading to apnea or hypoxemia, thus affecting sleep and quality of life (39,40). Obesity and PCOS are considered risk factors for obstructive sleep apnea (39,40). Like PCOS, there is an increased risk of insulin resistance with OSA, apart from other metabolic disturbances (40).
Research studies observed a high prevalence of inflammatory bowel syndrome (IBS) in PCOS patients, thus affecting their quality of life (41,48,49). Increased LH/FSH levels and stress appear to be the key factors for this high prevalence (48). Moreover, obesity may exacerbate sleep disorders and psychiatric illnesses in women who are simultaneously affected by PCOS and IBS (41).
Obese PCOS patients are susceptible to poor psychological health (12,50). Research indicates that anxiety, depression, and poor body image are more common in PCOS patients than in healthy women (11,50). PCOS-related symptomatology, such as menstrual irregularity, failure to conceive, and hirsutism, may make obese women vulnerable to emotional stress, mental health problems, and impaired psychological quality of life. A research study found that overweight women with PCOS-related infertility (who were trying to conceive and lose weight) had low scores of PCOS health-related quality of life (51).
B. The impact of weight management on women with Polycystic Ovary Syndrome and coexisting Obesity
Research has consistently shown a positive impact of weight loss in ameliorating metabolic, androgenic, and biochemical derangements in overweight and obese women with PCOS (6,33,34). Weight reduction benefits obese PCOS patients by lowering fasting blood glucose levels and optimizing fasting insulin, serum free-testosterone levels, lipid profile, and blood pressure control (52,53). Studies indicate that weight loss (attained through lifestyle intervention) improves various reproductive parameters related to menstrual cycles, ovulation, and pregnancy rate, in overweight and obese females affected with PCOS (35,54-58).
The weight control measures and their influence on PCOS management have been explicitly discussed in the literature. The beneficial effect of even a modest weight loss on metabolic and reproductive health cannot be overemphasized in PCOS (15,59-61). Research data indicate that modest weight loss (minimum of 5% of body weight) may alleviate endocrine, reproductive, and metabolic derangements in overweight/ obese women with PCOS, including improvement in hyperandrogenism, insulin resistance, and metabolic index (60-62).
Obesity is associated with adverse reproductive health consequences such as infertility, ovulation dysfunction, and miscarriages (63,64). A retrospective cohort study found weight loss (10% of the body weight) associated with significant improvement in pregnancy and live birth rates in overweight women with infertility (43). Hence, effective pre-pregnancy counselling for weight reduction should be an integral part of the management strategy of PCOS-related infertility in overweight and obese patients (32).
There is a high risk of anxiety, depression, and low self-esteem reported in women with PCOS (42). Research studies depict the beneficial impact of physical activity on psychological health in overweight and obese patients with PCOS (65,66).
Study indicates that overweight women experiencing PCOS-related infertility (while trying to conceive and achieve weight loss) tend to have poor eating behaviors and diet, thus making healthy weight goal difficult to achieve (51). Weight management is challenging in the patient population with PCOS and coexisting obesity, often leading to non-adherence and a high drop-out rate from weight control programs (4,67). This may also be attributed to the compromised lipolytic function of adipocytes under the androgenic effect (4,68). Future research must focus on androgen-mediated lipolysis dysfunction in PCOS and coexisting obesity.
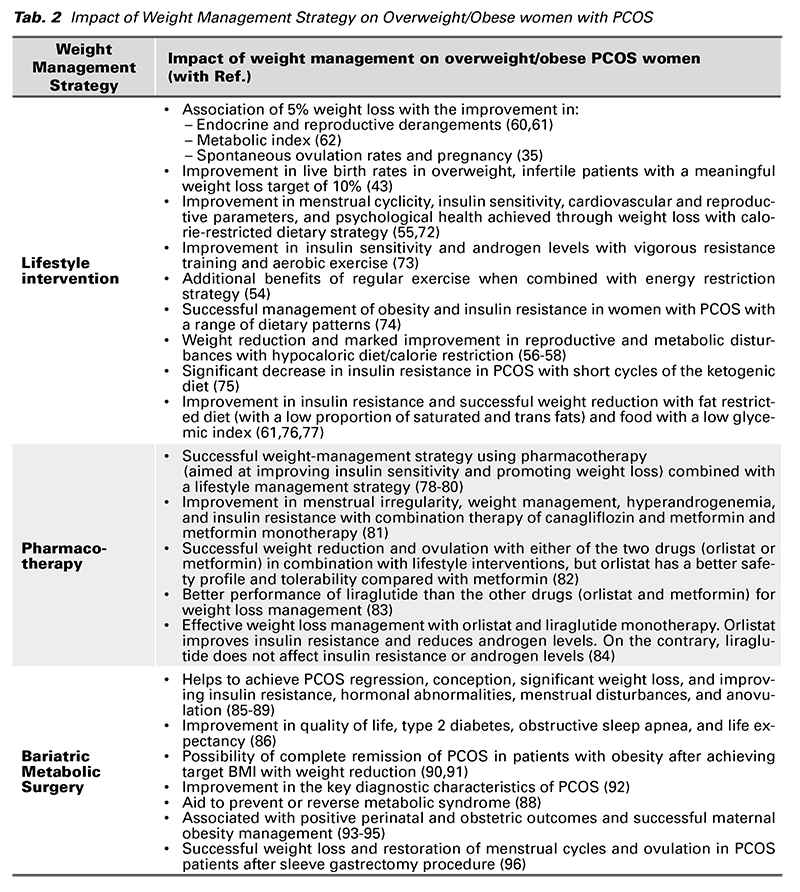
Weight reduction goals can be accomplished in PCOS through intensive, structured, and sustainable strategies aimed at reasonable weight reduction (21,53,59,69). Weight management programs incorporating cognitive behavioural therapy may help obese women with PCOS attain satisfactory and sustainable weight loss (70). Based on the current evidence, various weight management strategies include lifestyle intervention, pharmacotherapy, and bariatric surgery (71). Each strategy is prioritized, considering multiple factors such as the required magnitude of target weight loss, the timeline of fertility treatment, and comorbidities associated with PCOS and concurrent obesity (71). The impact of various weight management strategies on PCOS obese/ overweight women is briefly summarized in Table 2.
Lifestyle intervention can help overweight/obese women to achieve their target weight and favourable reproductive outcomes such as improvement in the menstrual cycles, ovulation, and pregnancy rate (35,54-58,97). Weight reduction strategy through lifestyle intervention requires three key components: Diet, exercise, and behavioral therapy (69). Weight loss program that integrates dietary approach with exercise should be tailored according to an individual’s body composition requirement and nutritional assessment (72,98). The effects of various diet plans are recently investigated and considered one of the beneficial non-pharmacological strategies for weight management of overweight and obese patients with PCOS (68,75,97). Dietary models recommending food consumption with a low glycemic index and low proportion of trans- and saturated fatty acids seem promising in combating insulin resistance and achieving weight reduction in women with PCOS (61,76,77). Nutrition and exercise can be complemented by short-term pharmacotherapy to treat insulin resistance and promote weight loss in the initial management of PCOS (78).
Appropriate exercise programs and dietary approaches may help to restore ovulation and regularize the menstrual cycles in approximately 49% of patients with polycystic ovary syndrome (74). Resistance or strength training may improve androgen levels and insulin sensitivity in women with PCOS (73). How- ever, extremely heavy exercise (more than 60 minutes per day) is not recommended for women with PCOS who seek fertility because of the increased risk of anovulation (99).
An effective multidisciplinary weight management program, using a combined approach of nutrition counselling, exercise, and cognitive behavioral therapy, may encourage overweight/obese women with PCOS to lose weight and maintain it long-term after initial weight reduction (70,100,101). Behavioral strategy and optimal psychological support are likely to improve the outcomes of weight management interventions (101). Such strategies should consider the nutritional and mental health requirements of reproductive-aged women and include stress management (101). Telehealth services can be utilized to provide mental health rehabilitation as a part of reproductive care and preconception counselling in women struggling with infertility (102).
Potential pharmacotherapy in PCOS-specific weight management may comprise insulin-sensitizing monotherapy and various pharmacologic therapy options to induce weight loss (79,81-84). In a setting of PCOS and coexistent obesity, an effective weightloss pharmacotherapy may improve spontaneous conception rates and response to fertility treatments such as ovulation induction and IVF (71). An integrated weight reduction strategy, with a combined approach of lifestyle intervention and pharmacotherapy, can help reduce body weight, insulin resistance and androgen levels in PCOS patients (79,80).
Bariatric metabolic surgery is one of the weight management options to achieve a target body mass index (BMI) and the required body weight in obese
patients with PCOS (85,90). This specific surgical intervention requires certain patient selection criteria for the recommendation and thorough assessment considering factors such as BMI, presence/absence of comorbidities, potential risks and benefits associated with the surgery, response to lifestyle intervention and medical therapy for weight management, and patient’s fertility plan (35,37,53,85,90,91). Patient preference and informed choice are essential for shared decision-making before bariatric surgery (103). Various bariatric surgery procedures have been described in the literature, such as Roux-en-Y gastric bypass, sleeve gastrectomy, and adjustable gastric band, to name a few (85).
Multiple research studies mention bariatric surgery as an effective weight-loss procedure and relate it with positive outcomes in selected cases of PCOS, including improvement in insulin resistance, metabolic syndrome-related features, hyperandrogenism, menstrual irregularity, ovulatory dysfunction, and complete remission of polycystic ovary syndrome (85-88,90-92,96). Limited evidence suggests improved chances of conception and fertility in PCOS patients after weight loss following bariatric surgery (86,89). While increasing evidence mentions the beneficial impact of bariatric surgery on obstetric and perinatal outcomes, research data also highlights a few maternal and neonatal complications observed in pregnancies after bariatric surgery (89,93-95). Large and sufficiently powered studies are required to reflect the bariatric surgery-related specific reproductive, maternal, and neonatal outcomes in women with PCOS and concurrent obesity (85,86).
More extensive research and RCTs are required to determine short- and long-term reproductive, endocrine, and metabolic outcomes using various weight loss strategies in overweight and obese females with PCOS. Qualitative research evidence can also help formulate PCOS-specific protocols for weight reduction and clinical practice guidelines for weight management in PCOS with concurrent obesity.
Conclusion
Given the increasing prevalence of the ongoing obesity pandemic, the prevalence of PCOS is likely to rise. Polycystic ovary syndrome and obesity, both conditions, affect women through multiple complex pathogenic mediating mechanisms. The clinical manifestations of PCOS associated with reproductive, metabolic, endocrine, cardiovascular, and psychological dysfunction tend to worsen with obesity. Hence, an effective weight reduction strategy can be considered a milestone in optimizing reproductive, endocrine, and metabolic outcomes in PCOS management of obese women.
Healthcare professionals must pursue a proactive approach to identify and timely manage both conditions, particularly in adolescent girls and young women of reproductive age. Preventing excessive weight gain from an early age should be an important part of a continuum of care. More robust interventional and methodological studies, including RCTs, are still required to examine the short- and long-term impacts of obesity on PCOS and determine the optimal magnitude of weight loss through effective obesity management programs in overweight and obese women with PCOS.
Funding: The author received no financial support for this article’s research, authorship, and publication.
Declaration of conflicting interests: The author declared no potential conflicts of interest concerning this article’s research, authorship, and publication.
Literature
- Azziz R. Introduction: Determinants of polycystic ovary syndrome. Fertil Steril. 2016;106(1):4-5, doi: 10.1016/j.fertnstert.2016.05.009
- Escobar-Morreale HF. Defining PCOS: A syndrome with an intrinsic heterogeneous nature. Polycystic Ovary Syndr. January 2022:3-13, doi: 10.1016/B978-0-12-823045-9.00012-2
- Çelik Ö, Köse MF. An overview of polycystic ovary syndrome in aging women. J Turkish Ger Gynecol Assoc. 2021;22(4):326-33, doi: 10.4274/jtgga.galenos.2021.2021.0077
- Barber TM, Hanson P, Weickert MO, Franks S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin Med Insights Reprod Heal. 2019;13, doi: 10.1177/1179558119874042
- Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94(2):106-113
- Barber TM, McCarthy MI, Wass JAH, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65(2):137-145, doi: 10.1111/j.1365-2265.2006.02587.x
- Li X, Yang D, Pan P, et al. The Degree of Menstrual Disturbance Is Associated With the Severity of Insulin Resistance in PCOS. Front Endocrinol (Lausanne). 2022;13:873726, doi: 10.3389/fendo.2022.873726
- Saadia Z. Follicle Stimulating Hormone (LH: FSH) Ratio in Polycystic Ovary Syndrome (PCOS) - Obese vs. Non- Obese Women. Med Arch (Sarajevo, Bosnia Herzegovina). 2020;74(4):289-293, doi: 10.5455/medarh.2020.74.289-293
- Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long- term follow-up: A retrospective cohort study. Clin Endocrinol (Oxf). 2000;52(5):595-600, doi: 10.1046/j.1365-2265.2000.01000.x
- Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;62:318- 325, doi: 10.1007/s12020-018-1692-3
- Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075-1091, doi: 10.1093/humrep/dex044
- Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2012;97(1):225-230.e2, doi: 10.1016/j.fertnstert.2011.10.022
- Karsten MDA, Wekker V, Groen H, et al. The role of PCOS in mental health and sexual function in women with obesity and a history of infertility. Hum Reprod Open. 2021;2021(4), doi: 10.1093/hropen/hoab038
- Saha L, Kaur S, Saha PK. Pharmacotherapy of polycystic ovary syndrome - An update. Fundam Clin Pharmacol. 2012;26(1):54-62, doi: 10.1111/j.1472-8206.2010.00916.x
- Hoeger KM. Obesity and lifestyle management in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50(1):277-294, doi: 10.1097/GRF.0b013e31802f54c8
- Vrbikova J, Hainer V. Obesity and Polycystic Ovary Syndrome. Obes Facts. 2009;2(1):26-35, doi: 10.1159/000194971
- Legro RS. The genetics of obesity lessons for polycystic ovary syndrome. Annals of the New York Academy of Sciences. 2000;900(1):193-202, doi: 10.1111/j.1749-6632.2000.tb06230.x
- Sam S. Obesity and Polycystic Ovary Syndrome. Obes Manag. 2007;3(2):69-73, doi: 10.1089/ obe.2007.0019
- Kujanpää L, Arffman RK, Vaaramo E, et al. Women with polycystic ovary syndrome have poorer work ability and higher disability retirement rate at midlife: a Northern Finland Birth Cohort 1966 study. Eur J Endocrinol. 2022;187(3):479-488, doi: 10.1530/EJE-22-0027
- Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618-637, doi: 10.1093/humupd/dms030
- Floyd R, Gryson R, Mockler D, Gibney J, Duggan SN, Behan LA. The Effect of Time-Restricted Eating on Insulin Levels and Insulin Sensitivity in Patients with Polycystic Ovarian Syndrome: A Systematic Review. Int J Endocrinol. 2022;2022:1-13, doi: 10.1155/2022/2830545
- Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020;105(8):e2695-e2709, doi: 10.1210/clinem/dgaa285
- Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: Insight into pathogenesis and a common association with insulin resistance. Clinical Medicine. 2015;15(6):s72-s76, doi: 10.7861/clinmedicine.15-6-s72
- Deswal R, Narwal V, Dang A, Pundir CS. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J Hum Reprod Sci. 2020;13(4):261-271, doi: 10.4103/jhrs.JHRS_95_18
- Hoeger K. Obesity and weight loss in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28(1):85-79, doi: 10.1016/S0889-8545(05)-70187-X
- Laitinen J, Taponen S, Martikainen H, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes. 2003;27:710-715, doi: 10.1038/sj.ijo.0802301
- Barber TM. Why are women with polycystic ovary syndrome obese? Br Med Bull. 2022;143(1):4- 15, doi: 10.1093/bmb/ldac007
- Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: Progress and paradoxes. Recent Prog Horm Res. 2001;56:295-308, doi: 10.1210/rp.56.1.295
- Homburg R. Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2008;22(2):261- 274, doi: 10.1016/j.bpobgyn.2007.07.009
- Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19(2):81-87, doi: 10.1097/MED.0b013e3283514e13
- Calcaterra V, Verduci E, Cena H, et al. Polycystic ovary syndrome in insulin‐resistant adolescents with obesity: The role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients. 2021;13(6):1848, doi: 10.3390/nu13061848
- Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10(3):267-280, doi: 10.1093/humupd/dmh018
- Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16:22, doi: 10.1186/s12958-018-0336-z
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347-364, doi: 10.1530/REP-09-0568
- Motta AB. The Role of Obesity in the Development of Polycystic Ovary Syndrome. Curr Pharm Des. 2012;18(17):2482-2491, doi: 10.2174/13816128112092482
- Cunha A, Póvoa AM. Infertility management in women with polycystic ovary syndrome: a review. Porto Biomed J. 2021;6(1):e116, doi: 10.1097/j.pbj.0000000000000116
- Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687- 708, doi: 10.1093/humupd/dmw025
- Navaratnarajah R, Pillay O, Hardiman P. Polycystic Ovary Syndrome and Endometrial Cancer. Semin Reprod Med. 2008;26(1):062-071, doi: 10.1055/s-2007-992926
- Kahal H, Kyrou I, Tahrani AA, Randeva HS. Obstructive sleep apnoea and polycystic ovary syndrome: A comprehensive review of clinical interactions and underlying pathophysiology. Clin Endocrinol (Oxf). 2017;87(4):313-319, doi: 10.1111/cen.13392
- Sam S, Tasali E. Role of obstructive sleep apnea in metabolic risk in PCOS. Curr Opin Endocr Metab Res. 2021;17:46-51, doi: 10.1016/j.coemr.2021.01.002
- Tseng PH, Chiu HM, Tu CH, Wu MS, Ho HN, Chen MJ. Obesity Exacerbates Irritable Bowel Syndrome- Related Sleep and Psychiatric Disorders in Women With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2021;12:779456, doi: 10.3389/fendo.2021.779456
- Rashid R, Mir SA, Kareem O, et al. Polycystic ovarian syndrome-current pharmacotherapy and clinical implications. Taiwan J Obstet Gynecol. 2022;61(1):40-50, doi: 10.1016/j.tjog.2021.11.009
- Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril. 2014;101(5):1400-1403, doi: 10.1016/j.fertnstert.2014.01.036
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748-758, doi: 10.1093/humupd/dmu012
- Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control. 2010;21:2303- 2308, doi: 10.1007/s10552-010-9658-7
- Tokmak A, Kokanali MK, Guzel AI, Kara A, Topcu HO, Cavkaytar S. Polycystic ovary syndrome and risk of endometrial cancer: A mini-review. Asian Pacific J Cancer Prev. 2014;15(17):7011-7014, doi: 10.7314/APJCP.2014.15.17.7011
- Sidorkiewicz I, Jóźwik M, Niemira M, Krętowski A. Insulin resistance and endometrial cancer: Emerging role for microRNA. Cancers (Basel). 2020;12(9):2559, doi: 10.3390/cancers12092559
- Bazarganipour F, Taghavi SA, Asemi Z, et al. The impact of irritable bowel syndrome on health -related quality of life in women with polycystic ovary syndrome. Health Qual Life Outcomes. 2020;18:226, doi: 10.1186/s12955-020-01428-7
- Kałużna M, Kompf P, Wachowiak-Ochmańska K, et al. Are patients with polycystic ovary syndrome more prone to irritable bowel syndrome? Endocr Connect. 2022;11(4):e210309, doi: 10.1530/EC-21-0309
- Blay SL, Aguiar JVA, Passos IC. Polycystic ovary syndrome and mental disorders: A systematic review and exploratory meta-analysis. Neuropsychiatr Dis Treat. 2016;2016(12):2895-2903, doi: 10.2147/NDT.S91700
- Turner-Mcgrievy G, Davidson CR, Billings DL. Dietary intake, eating behaviors, and quality of life in women with polycystic ovary syndrome who are trying to conceive. Hum Fertil. 2015;18(1):16- 21, doi: 10.3109/14647273.2014.922704
- Abdulkhalikova D, Sustarsic A, Vrtačnik Bokal E, Jancar N, Jensterle M, Burnik Papler T. The Lifestyle Modifications and Endometrial Proteome Changes of Women With Polycystic Ovary Syndrome and Obesity. Front Endocrinol (Lausanne). 2022;13:888460, doi: 10.3389/fendo.2022.888460
- Saydam BO, Yildiz BO. Weight management strategies for patients with PCOS: current perspectives. Expert Rev Endocrinol Metab. 2021;16(2):49- 62, doi: 10.1080/17446651.2021.1896966
- Thomson RL, Buckley JD, Brinkworth GD. Exercise for the treatment and management of overweight women with polycystic ovary syndrome: A review of the literature. Obes Rev. 2011;12(5):e202-e210, doi: 10.1111/j.1467-789X.2010.00758.x
- Moran LJ, Noakes M, Clifton PM, Tomlinson L, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(2):812- 819, doi: 10.1210/jc.2002-020815
- Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004;81(3):630-637, doi: 10.1016/j.fertnstert.2003.08.023
- Kiddy DS, Hamilton‐Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36(1):105-111, doi: 10.1111/j.1365-2265.1992.tb02909.x
- Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77-87, doi: 10.1093/ajcn/84.1.77
- Pelletier L, Baillargeon JP. Clinically significant and sustained weight loss is achievable in obese women with polycystic ovary syndrome followed in a regular medical practice. Fertil Steril. 2010;94(7):2665-2669, doi: 10.1016/j.fertnstert.2010.02.047
- Rondanelli M, Perna S, Faliva M, Monteferrario F, Repaci E, Allieri F. Focus on metabolic and nutritional correlates of polycystic ovary syndrome and update on nutritional management of these critical phenomena. Arch Gynecol Obstet. 2014;290:1079-1092, doi: 10.1007/s00404-014-3433-z
- Faghfoori Z, Fazelian S, Shadnoush M, Goodarzi R. Nutritional management in women with polycystic ovary syndrome: A review study. Diabetes Metab Syndr Clin Res Rev. 2017;11(Supp.1):S- 429-S432, doi: 10.1016/j.dsx.2017.03.030
- Kim CH, Lee SH. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life. 2022;12(2):308, doi: 10.3390/life12020308
- Marsh CA, Hecker E. Maternal obesity and adverse reproductive outcomes: Reducing the risk. Obstet Gynecol Surv. 2014;69(10):622-628, doi: 10.1097/ogx.0000000000000115
- Moran LJ, Dodd J, Nisenblat V, Norman RJ. Obesity and reproductive dysfunction in women. Endocrinol Metab Clin North Am. 2011;40(4):895- 906, doi: 10.1016/j.ecl.2011.08.006
- Liao LM, Nesic J, Chadwick PM, Brooke-Wavell K, Prelevic GM. Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: A pilot investigation. Gynecol Endocrinol. 2008;24(10):555-561, doi: 10.1080/09513590802288226
- Thomson RL, Buckley JD, Lim SS, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril. 2010;94(5):1812-1816, doi: 10.1016/j.fertnstert.2009.11.001
- Moran LJ, Lombard CB, Lim S, Noakes M, Teede HJ. Polycystic ovary syndrome and weight management. Women’s Health. 2010;6(2):271-283, doi: 10.2217/whe.09.89
- Cincione RI, Losavio F, Ciolli F, et al. Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome. Int J Environ Res Public Health. 2021;18(23):12490, doi: 10.3390/ijerph182312490
- Moran LJ, Noakes M, Clifton P, et al. Predictors of lifestyle intervention attrition or weight loss success in women with polycystic ovary syndrome who are overweight or obese. Nutrients. 2019;11(3):492, doi: 10.3390/nu11030492
- Jiskoot G, Benneheij SH, Beerthuizen A, et al. A three-component cognitive behavioural lifestyle program for preconceptional weight-loss in women with polycystic ovary syndrome (PCOS): A protocol for a randomized controlled trial. Reprod Health. 2017;14:34, doi: 10.1186/s12978-017-0295-4
- Hazlehurst JM, Singh P, Bhogal G, Broughton S, Tahrani AA. How to manage weight loss in women with obesity and PCOS seeking fertility? Clin Endocrinol (Oxf). 2022;97(2):208-216, doi: 10.1111/cen.14726
- Moran LJ, Ko H, Misso M, et al. Dietary Composition in the Treatment of Polycystic Ovary Syndrome: A Systematic Review to Inform Evidence-Based Guidelines. J Acad Nutr Diet. 2013;113(4):520- 545, doi: 10.1016/j.jand.2012.11.018
- Shele G, Genkil J, Speelman D. A systematic review of the effects of exercise on hormones in women with polycystic ovary syndrome. J Funct Morphol Kinesiol. 2020;5(2):35, doi: 10.3390/jfmk5020035
- Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(9):3373-3380, doi: 10.1210/jc.2008-0751
- Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:401, doi: 10.1186/s12967-020-02277-0
- Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2019;2019:4386401, doi: 10.1155/2019/4386401
- Che X, Chen Z, Liu M, Mo Z. Dietary Interventions: A Promising Treatment for Polycystic Ovary Syndrome. Ann Nutr Metab. 2021;77:313-323, doi: 10.1159/000519302
- Farshchi H, Rane A, Love A, Kennedy RL. Diet and nutrition in polycystic ovary syndrome (PCOS): Pointers for nutritional management. J Obstet Gynaecol (Lahore). 2007;27(8):762-773, doi: 10.1080/01443610701667338
- Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2016;22(3):408-409, doi: 10.1093/humupd/dmv063
- Panidis D, Tziomalos K, Papadakis E, et al. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2014;80(3):432-438, doi: 10.1111/cen.12305
- Zhang J, Xing C, Cheng X, He B. Canagliflozin combined with metformin versus metformin monotherapy for endocrine and metabolic profiles in overweight and obese women with polycystic ovary syndrome: A single-center, open-labeled prospective randomized controlled trial. Front Endocrinol (Lausanne). 2022;13:1003238, doi: 10.3389/fendo.2022.1003238
- Kumar P, Arora S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci. 2014;7(4):255-261, doi: 10.4103/0974-1208.147492
- Wang FF, Wu Y, Zhu YH, et al. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta -analysis. Obes Rev. 2018;19(10):1424-1445, doi: 10.1111/obr.12720
- Chatzis P, Tziomalos K, Pratilas GC, Makris V, Sotiriadis A, Dinas K. The Role of Antiobesity Agents in the Management of Polycystic Ovary Syndrome. Folia Med (Plovdiv). 2018;60(4):512- 520, doi: 10.2478/folmed-2018-0036
- Lee R, Joy Mathew C, Jose MT, Elshaikh AO, Shah L, Cancarevic I. A Review of the Impact of Bariatric Surgery in Women With Polycystic Ovary Syndrome. Cureus. 2020;12(10):e10811, doi: 10.7759/cureus.10811
- Butterworth J, Deguara J, Borg CM. Bariatric Surgery, Polycystic Ovary Syndrome, and Infertility. J Obes. 2016;2016:1871594, doi: 10.1155/2016/1871594
- Skubleny D, Switzer NJ, Gill RS, et al. The Impact of Bariatric Surgery on Polycystic Ovary Syndrome: a Systematic Review and Meta-analysis. Obes Surg. 2016;26:169-176, doi: 10.1007/s11695-015-1902-5
- Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes. 2012;3(4):71-79, doi: 10.4239/wjd.v3.i4.71
- Benito E, Gómez-Martin JM, Vega-Piñero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105(9):e3384-e3391, doi: 10.1210/clinem/dgaa439
- Hu L, Ma L, Xia X, et al. Efficacy of Bariatric Surgery in the Treatment of Women With Obesity and Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2022;107(8):e3217-e3229, doi: 10.1210/clinem/dgac294
- Escobar-Morreale HF, Botella-Carretero JI, Álvarez- Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90(12):6364-6369, doi: 10.1210/jc.2005-1490
- Christ JP, Falcone T. Bariatric Surgery Improves Hyperandrogenism, Menstrual Irregularities, and Metabolic Dysfunction Among Women with Polycystic Ovary Syndrome (PCOS). Obes Surg. 2018;28:2171-2177, doi: 10.1007/s11695-018-3155-6
- Maslin K, Douek I, Greenslade B, Shawe J. Nutritional and perinatal outcomes of pregnant women with a history of bariatric surgery: a case series from a UK centre. J Hum Nutr Diet. 2020;33(3):386-395, doi: 10.1111/jhn.12718
- Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol. 2018;218(6):573-580, doi: 10.1016/j.ajog.2018.02.003
- Al-Nimr RI, Hakeem R, Moreschi JM, et al. Effects of Bariatric Surgery on Maternal and Infant Outcomes of Pregnancy—An Evidence Analysis Center Systematic Review. J Acad Nutr Diet. 2019;119(11):1921-1943, doi: 10.1016/j.jand.2019.02.008
- Wang K, Jiang Q, Zhi Y, et al. Contrasting sleeve Gastrectomy with lifestyle modification therapy in the treatment of polycystic ovary syndrome. J Laparoendosc Adv Surg Tech. 2015;25(6):493- 498, doi: 10.1089/lap.2014.0511
- Mehrabani HH, Salehpour S, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic- load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: A randomized controlled intervention study. J Am Coll Nutr. 2012;31(2):117-125, doi:10.1080/07315724.2012.10720017
- Barrea L, Arnone A, Annunziata G, et al. Adherence to the mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients. 2019;11(10):2278, doi: 10.3390/nu11102278
- Hakimi O, Cameron LC. Effect of Exercise on Ovulation: A Systematic Review. Sport Med. 2017;47:1555-1567, doi: 10.1007/s40279-016-0669-8
- Jiskoot G, Timman R, Beerthuizen A, Dietz de Loos A, Busschbach J, Laven J. Weight Reduction Through a Cognitive Behavioral Therapy Lifestyle Intervention in PCOS: The Primary Outcome of a Randomized Controlled Trial. Obesity. 2020;28(11):2134-2141, doi: 10.1002/oby.22980
- Brennan L, Teede H, Skouteris H, Linardon J, Hill B, Moran L. Lifestyle and Behavioral Management of Polycystic Ovary Syndrome. J Women’s Heal. 2017;26(8):836-848, doi: 10.1089/jwh.2016.5792
- Ghafoor S. Maternal-Fetal Care and Telehealth in the Context of COVID-19 Pandemic: A Narrative Review. Int J Obstet Gynaecol Res. 2021;7(1):852-870, http://www.ijogr.com/2021/maternal-fetal-care-and-telehealth-in-the-context-of-covid-19-pandemic-a-narrative-review/
- Lee YC, Wu WL. Shared decision making and choice for bariatric surgery. Int J Environ Res Public Health. 2019;16(24):4966, doi: 10.3390/ijerph16244966











 Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.
Official publication of the Czech Society of Ultrasound in Obstetrics and Gynecology.



